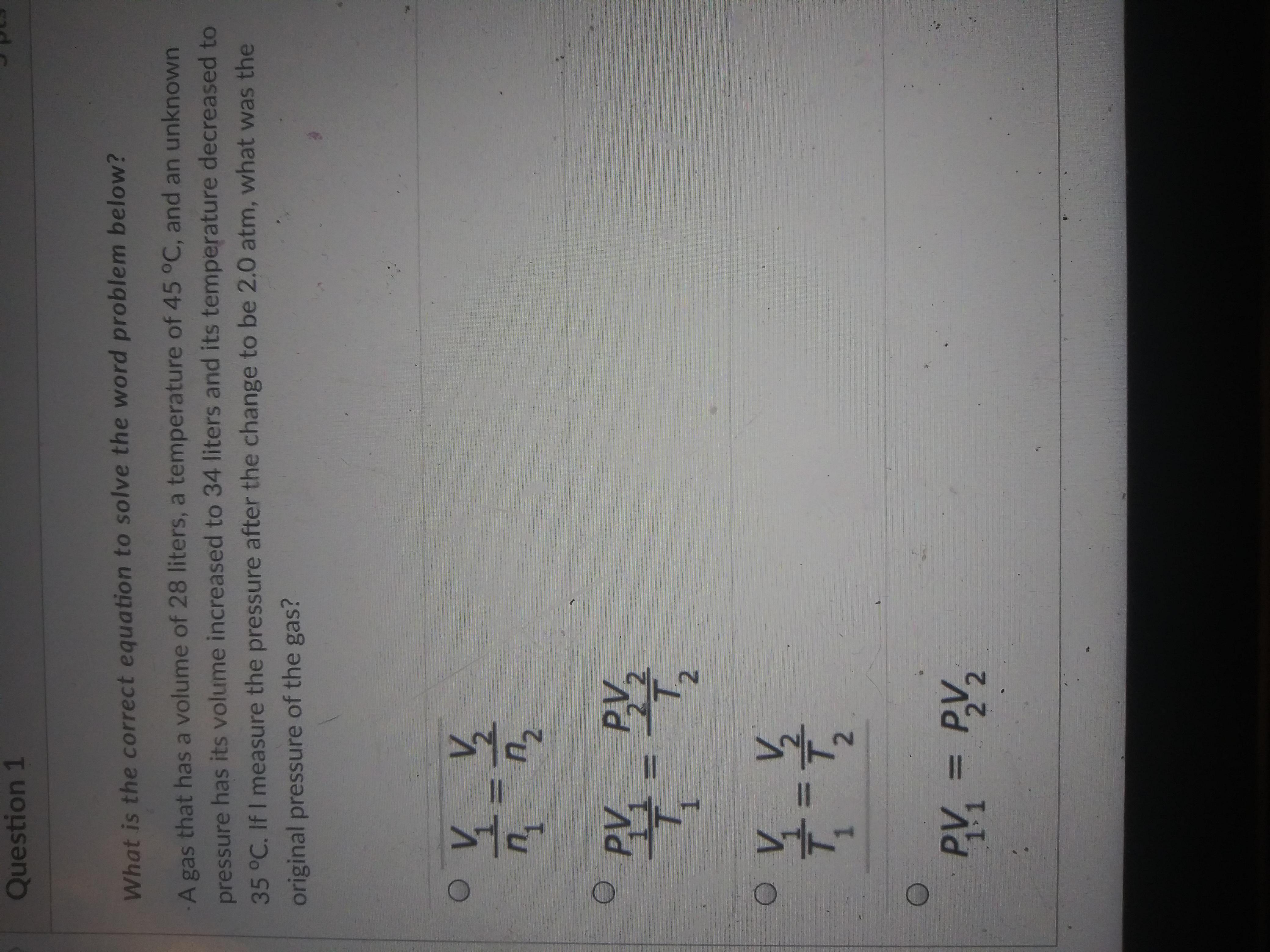
Answers
Answer:
D
Explanation:
Related Questions
H2SO4 + 2NaOH - H2O + Na2SO4
Use the balanced chemical equation to solve the following problems. Show all work and answer with the correct units and significant figures.
a. How many moles of sodium sulfate are produced if 13 g of sulfuric acid is used in the reaction?
b. How many molecules of water are produced if 2.0 g of sodium sulfate is produced in the above reaction?
c. What mass of sulfuric acid is required to completely react with 3.4 x 1024 formula units of sodium hydroxide?
Please help
Answers
Explanation:
The balanced equation for the reaction is given as;
2NaOH + H2SO4 → Na2SO4 + 2H2O
a. How many moles of sodium sulfate are produced if 13 g of sulfuric acid is used in the reaction?
From the reaction;
1 mol of H2SO4 produces 1 mol of Na2SO4
Converting 13g of sulphuric acid to mol;
Number of moles = Mass / Molar mass
Number of moles = 13g / 98.0785 g/mol
Number of moles = 0.1325 mol
Since the mol ratio is 1: 1. This means
0.1325 mol of sodium sulfate would be formed.
b. How many molecules of water are produced if 2.0 g of sodium sulfate is produced in the above reaction?
From the reaction;
1 mol of Na2SO4 is produced alongside 2 mol of H2O
Converting 2g of sodium sulfate mol;
Number of moles = Mass / Molar mass
Number of moles = 2g / 142.04 g/mol
Number of moles = 0.01408 mol
Since the mol ratio is 1: 2. This means
0.02816 mol of water would be formed.
1 mol of water = 6.022×10^23 molecules
0.02816 mol = x
Solving for x;
x = 1.696 ×10^22 molecules
c. What mass of sulfuric acid is required to completely react with 3.4 x 1024 formula units of sodium hydroxide?
From the reaction;
1 mol of H2SO4 reacts with 2 mol of NaOH
1 mol = 6.022×10^23 units
x mol = 3.4 x 10^24 units
Solving for x;
x = 5.646 mol of NaOH
Since the mole ratio is 1:2;
5.646 mol / 2 = 2.823 mol of sulphuric acid
Converting too mass;
Mass = Number of moles * Molar mass
Mass = 2.823 mol * 98.0785 g/mol
Mass = 276.88 g
What is produced when concentrated aqueous sodium chloride is electrolysed?
Answers
You’re welcome!! Have a good day/night :)
9.
Which of the following samples contains the greatest number of atoms?
a. 1 mole of CO2
c. 3 moles of N20
b. b. 2 moles of He
d. 4 moles of CO
Answers
Answer:
N2O.
Explanation:
Hello there!
In this case, according to the Avogadro's number, we can compute the number of atoms, taking into account that CO2 has three moles of atoms, N2O has three moles of atoms, He one mole of atoms and CO two moles of atoms:
[tex]atoms_{CO_2}=1molCO_2*\frac{3*6.022x10^{23}atoms}{1molCO_2}=1.81x10^{24}atoms\\\\atoms_{N_2O}=3molN_2O*\frac{3*6.022x10^{23}atoms}{1molN_2O}=5.4x10^{24}atoms\\\\atoms_{He}=2molHe*\frac{1*6.022x10^{23}atoms}{1molHe}=1.2x10^{24}atoms\\\\atoms_{CO}=4molCO*\frac{2*6.022x10^{23}atoms}{1molCO}=4.82x10^{24}atoms[/tex]
Thus, we infer that 3 moles of N2O have the greatest number of atoms.
How many molecules are in 450.0 grams of aluminum fluoride(AIF3)?
Answers
Answer:
It would be exactly 5.3586262014272155. But if you were to round it up it would be 5.35.
*PLEASE HELP*
Write the word equation and the balanced symbol equation for the following reactions
1.Sulfuric acid reacting with sodium carbonate
2.Hydrochloric acid reacting with magnesium hydrogencarbonate
Answers
Answer:
1. H₂SO₄ + Na₂CO₃ ⇒ Na₂SO₄ + H₂O + CO₂
sulfuric acid + sodium carbonate ⇒ sodium sulfate + water + carbon dioxide
2. 2 HCl + Mg(HCO₃)₂ ⇒ MgCl₂ + 2 H₂O + 2 CO₂
hydrochloric acid + magnesium hydrogencarbonate ⇒ magnesium chloride + water + carbon dioxide
Explanation:
1. Sulfuric acid is H₂SO₄. Sodium carbonate is Na₂CO₃.
H₂SO₄ + Na₂CO₃ ⇒ Na₂SO₄ + H₂O + CO₂
sulfuric acid + sodium carbonate ⇒ sodium sulfate + water + carbon dioxide
2. Hydrochloric acid is HCl. Magnesium hydrogencarbonate is Mg(HCO₃)₂.
2 HCl + Mg(HCO₃)₂ ⇒ MgCl₂ + 2 H₂O + 2 CO₂
hydrochloric acid + magnesium hydrogencarbonate ⇒ magnesium chloride + water + carbon dioxide
In the process of performing a spectrophotometric determination of iron, an analyst prepares a calibration curve using a single-beam spectrophotometer. After preparing the calibration curve, the analyst drops and breaks the cuvette. The analyst acquires a new cuvette, measures the absorbance of the sample, and determines the %w/w Fe in the sample. Does the change in cuvette lead to a determinate error in the analysis
Answers
Answer:
Yes.
Explanation:
Yes, the change in cuvette lead to a determinate error in the analysis if the different cuvette is used for the analysis because the amount of liquid sample that is used has different volume. If both cuvette are of the same type and has no difference in their structure and size then there is error occurs in the analysis but if both cuvette are different from one another then the error will occur in the analysis. because the amount of liquid that is used has different volume.
PLZ HELP THIS IS DUE TODAY!!!!!!
Which statement BEST describes the motion of the molecules of a solid object
A They vibrate in place within a fixed volume.
B They move around freely within a fixed volume.
C They vibrate in place and take the shape of a container.
D They move around freely and take the shape of a container.
Answers
The first one is for gas, so it's wrong. The second one is for liquids. And finally, the third one is right.
Which of the following statements best describes the number of neutrons in an atom?
It is the same as the element's atomic number.
It is equal to the sum of atomic number and average atomic mass.
it is the same as the average atomic mass.
it is equal to the difference between atomic number and atomic mass number.
Answers
Answer:
its the last one
Explanation:
What is the mass of 500 trillion (5.0 x 10'4)
molecules of water?
Answers
When a hydrocarbon fuel is burned, almost all of the carbon in the fuel burns completely to form CO2 (carbon dioxide), which is the principal gas causing the greenhouse 102 ENERGY, ENERGY TRANSFER effect and thus global climate change. On average, 0.59 kg of CO2 is produced for each kWh of electricity generated from a power plant that burns natural gas. A typical new household refrigerator uses about 700 kWh of electricity per year. Determine the amount of CO2 production that is due to the refrigerators in a city with 300,000 households.
Answers
Answer:
1.239 * 10^8 Kg
Explanation:
Since all the electricity consumed comes from natural gas;
amount of electricity consumed = 700 * 300,000 = 2.1 * 10^8 kWh
So, amount of CO2 consumed is given by;
amount of electricity consumed * amount of CO2 per kWh
Hence,
Amount of CO2 = 2.1 * 10^8 kWh * 0.59 = 1.239 * 10^8 Kg
which statement explains this observation?
Answers
Answer:
should be D. Hope this helpsss!
How many formula units make up 25.8g of magnesium chloride (MgCl2)? Express the number of formula units numerically.
Answers
Answer:
Explanation:
Kandndkjwnww
In the UNBALANCED chemical reaction for the combustion of acetylene (used in welding torches), determine at standard temperature and pressure, how many liters of
H2O gas are produced if 12 liters of Oxygen gas are completely consumed?
__C2H2 + __O2 —> __CO2 + __H2O
(Please help! Random answers for points will be reported)
Answers
Answer:
2 C2H2 + 5 O2 = 4 CO2 + 2 H2O
I've checked this multiple times this should be it
A molecule is the smallest part of a ?
Answers
Answer:
A molecule is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms.
Explanation:
Answer:
A molecule is the smallest part of an element, sorry if im incorrect
number of molecules in lithium oxide
4li2O
Answers
Answer:
Explanation:
Lithium is in group 1 so there's 1 outermost shell electron.
Oxygen is in group 6 so there's 6 outermost shell electron.
To react lithium with oxygen, we need 2 lithium and 1 oxygen
Each lithium transfer 1 electron to the oxygen and oxygen gain 2 electrons.
[tex]Li_{2}O\\[/tex] formed.
If there's 4 lithium oxide, 4 molecules will be there.
If you mean how many ATOMS are there, there will be 3×4=12 atoms.
Guanidin HNC(NH2)2 is a fertilizer. What is the percent by mass of nitrogen in the fertilizer
Answers
Answer:
71.1%. , is a fertilizer.
The total mass of the compound guanidine is 59 g/mo. It contains 3 nitrogen atoms. Hence, the percent composition of nitrogen in this fertilizer is 71.18 %.
What is mass percent ?The mass percent or percent composition of an element in a compound is the measure of amount of that element in 100 g of the compound. Thus, it is the ratio of mass of that element in the compound to the total mass multiplied by 100.
mass percent = mass of the element in the sample/ total mass × 100
The given compound is guanidine with the formula, HNC(NH2)₂ .
atomic mass of N = 14 g/mol
mass of carbon = 12 g/mol
hydrogen = 1 g/mol.
Then total mass of guanidine = 14 + 12 + 1 + 2(2 + 14) = 59 g/mol.
there are 3 nitrogen.
mass of three nitrogen = 42 g.
Then mass percent of N in guanidine = 42 g/ 59 g × 100= 71.1%.
Therefore, the percent by mass of nitrogen in guanidine is 71.18%.
Find more on mass percent:
https://brainly.com/question/9904990
#SPJ6
Is the number of total molecules on the left side of a balanced equation always equal to the number of total molecules on the right side of the equation
Answers
Answer:
No
Explanation:
No, but the total mass of reactants must equal the total mass of products to be a balanced equation.
Example: Consider the following reaction ...
3H₂ + N₂ => 2NH₃ and 'amu' is atomic mass units (formula weights from periodic table)
In terms of molecules, there are 4 molecules on the left (3 molecular hydrogens (H₂) and 1 molecular nitrogen (N₂) and 2 molecules of ammonia on the right side of equation arrow. ∑reactant molecules ≠ ∑product molecules.
In terms of mass of reactants & mass of products, the 3H₂ + N₂ => 6amu + 28amu = 34amu & mass of products (2NH₃) => 2(14amu) + 6(1amu) = 34amu for sum of product masses.
∑mass reactants = ∑mass products <=> 34amu = 34amu.
The expression '∑mass reactants = ∑mass products' as applied to chemical equations is generally known as 'The Law of Mass Balance'.
Assume that the ratio of moles of Cl to moles of Cu are 2.39. If we can represent the formula by CuClx, what is the value for x? Round your answer to the nearest whole number. Do NOT include units. So, if you think the formula is CuCl6, you enter the number 6.
Answers
Answer:
x=2.
Explanation:
Hello there!
In this case, since we have a mole ratio of moles of Cl to moles of Cu as 2.39, it is possible to write the following:
[tex]\frac{2.39molCl}{1molCu}[/tex]
Thus, since the two possible copper chlorides are CuCl and CuCl₂, we can therefore infer that x=2 since it is close but not as close as it should to the whole number 2.
Best regards!
HELP ME
Elements in a vertical column on the Periodic Table have similar properties and are all members of the same
of elements.
А
molecule
B
phase
С
atom
D
group
Answers
Answer:
D group
................
HELLPPPP PLZZZ
many cars use electricity stored in lithium ion batteries as an alternative fuel. While there are many positive benefits to this technology, what negative impacts do they present? A) Reduces air pollution B) Decreases carbon emissions C) Disposal of spent batteries D) Cut dependence on fossil fuels
Answers
Answer:
C
Explanation:
is this one ok? XD
What is the molar mass of H2CO3?
(Molar mass of H = 1.0079 g/mol; C = 12.010 g/mol; O = 15.999 g/mol)
29.02 g/mol
46.04 g/mol
62.02 g/mol
72.08 g/mol
Answers
Answer:
62.02 g/mol
62.03 g/mol
Explanation:
62.03 g/mol
The molar mass of H2CO3 is 62.02 g/mol
What is molar mass?The molar mass of the compound is the sum of molar mass of indivitual atom multiple by number of that atom.
Calculation of molar mass of H2CO3 ,
Molar mass of H2CO3 = Molar mass of H*2 + Molar mass of C + Molar mass of O*3
Molar mass of H2CO3 = 1.0079*2 + 12.010 + 15.999*3
Molar mass of H2CO3 = 2.0158 + 12.010 + 47.997
Molar mass of H2CO3 = 62.02 g/mol
To learn more about Molar mass here.
https://brainly.com/question/12127540
#SPJ3
How many grams of sulfur is there in 10.8 grams of sodium sulfate?
please help
Answers
Answer:
2.44 g S
Explanation:
One mole of Na2SO4 contains 1 mol of S.
Step 1: Find out the moles of Na2SO4:
10.8 g Na2SO4/MM Na2SO4
Step2: Find out the moles of S:
moles S = moles Na2SO4
Step 3: Find out the mass of S.
mass S = moles S x MM S
Dimensional analysis:
? g S = 10.8 g Na2SO4 x (1mol Na2SO4/MM Na2SO4) x (1mol S/1mol Na2SO4) x (MM S/ 1 mol S) = (10.8/142.04)x32.1 = 2.44 g S
Select a reasonable explanation to account for the differences. There may be more than one possible reason that makes sense, just select one of them. It is possible not all of the water was evaporated from the sand, causing the recovered mass to be higher. It is possible not all of the water was evaporated from the sand, causing the recovered mass to be lower. While drying the NaCl, the liquid boiled and some splattered out of the evaporating dish, causing the recovered mass to be lower. While drying the NaCl, the liquid boiled and some splattered out of the evaporating dish, causing the recovered mass to be higher. There was no difference in recovered and original mass, so there is no difference to account for.
Answers
Answer:
It is possible not all of the water was evaporated from the sand, causing the recovered mass to be higher.
Explanation:
If not all the water is evaporated from the sand so the recovered mass to be higher than the sand from which more water is evaporated because evaporation of little amount of water causes less decrease in the recovered mass as compared to that sand from which more water is evaporated so more decrease occur in the recovered mass.
PLEASE PLEASE I do not understand may someone kindly help me pls
Answers
Answer:
It can be verified since the information came from a government article. Or you could trust the magzine/general article if its from a well known article. Explanation:
you get the point, you can add on to this. I can't make it the best since im not in your class learning. Good luck
Calculate each of the following quantities:
a) Molarity of a solution prepared by diluting 27.0 cm3
of 0.150 M potassium chloride to
150.0 cm3
b) Molarity of a solution prepared by diluting 35.71 cm3
of 0. 0756 M ammonium
sulfate to 500 cm3
c) Final volume of a 0.05M solution prepared by diluting 10.0 cm3
of 0.155 M lithium
carbonate with water
Answers
Answer:
A. 0.027 M
B. 0.0054 M
C. 31 cm³
Explanation:
A. Determination of the final concentration (Molarity)
Initial Volume (V₁) = 27 cm³
Initial concentration (C₁) = 0.150 M
Final volume (V₂) = 150 cm³
Final congratulation (C₂) =?
C₁V₁ = C₂V₂
0.150 × 27 = C₂ × 150
4.05 = C₂ × 150
Divide both side by 150
C₂ = 4.05 / 150
C₂ = 0.027 M
Thus, the final concentration of the solution is 0.027 M
B. Determination of the final concentration (Molarity)
Initial Volume (V₁) = 35.71 cm³
Initial concentration (C₁) = 0.0756 M
Final volume (V₂) = 500 cm³
Final congratulation (C₂) =?
C₁V₁ = C₂V₂
0.0756 × 35.71 = C₂ × 500
Divide both side by 500
C₂ = (0.0756 × 35.71) / 500
C₂ = 0.0054 M
Thus, the final concentration of the solution is 0.0054 M
C. Determination of the final volume.
Initial Volume (V₁) = 10 cm³
Initial concentration (C₁) = 0.155 M
Final congratulation (C₂) = 0.05 M
Final volume (V₂) =?
C₁V₁ = C₂V₂
0.155 × 10 = 0.05 × V₂
1.55 = 0.05 × V₂
Divide both side by 0.05
V₂ = 1.55 / 0.05
V₂ = 31 cm³
Thus, the final volume of the solution is 31 cm³
GUYS PLEASE HELP ME I BEG YOU
Answers
Answer:
an object is a rest upon a table
how many significant digits are in the number 1.0 × 10^3?
Answers
1•10^3 add three 0’s
The following image is a prediction of how an earthquake would spread from the epicenter (marked by a star). Red indicates the
worst areas, and green indicates the places where there is the least shaking. Water is colored blue. Consider a seismic wave-an
earthquake that starts in the middle of the red area and travels outward along the surface of the earth.
Image courtesy of U.S. Geological Survey
Department of the Interio/
USOS
Point A is the same distance from the epicenter as point B. Using what you know about the movement of waves, how would you
expect the seismic waves to be different at point A and point B?
OA.
The waves would be larger at point A than at point B.
OB. The waves would be exactly the same at points A and B.
OC. The waves would arrive at point B before they arrived at point A.
OD
The ground would shake up and down at point A but back and forth at point B.
Answers
Answer:
its B
Explanation:
The earthquakes are occurring more in the land region of earth surface. Hence, the waves would arrive at point B before they arrived at point.
What is an earthquake?When two layers of the ground abruptly slide past one another, an earthquake results. The fault or fault plane is the area where they slide. The epicenter is the point on the earth's surface that is directly above the hypocenter, which is where the earthquake begins under the surface.
The energy that would typically force the blocks to move past one another is being saved up while the fault edges are glued together and the rest of the block is moving.
All that accumulated energy is released when the force of the sliding blocks ultimately displaces the resistance of the sharp edges of the fault and causes it to unstick. Like ripples on a pond, the energy radiates from the fracture in all directions as seismic waves. Therefore, point B in the land surface will first experience the seismic waves.
To find more on earthquakes, refer here:
https://brainly.com/question/29500066
#SPJ2
(12 points) please please help me thanks
Answers
Answer: i think its the thired one
Explanation:
Hardness is the measure of a mineral's resistance to scratching.
True
False
Answers
Answer:
The answer to the question is true.