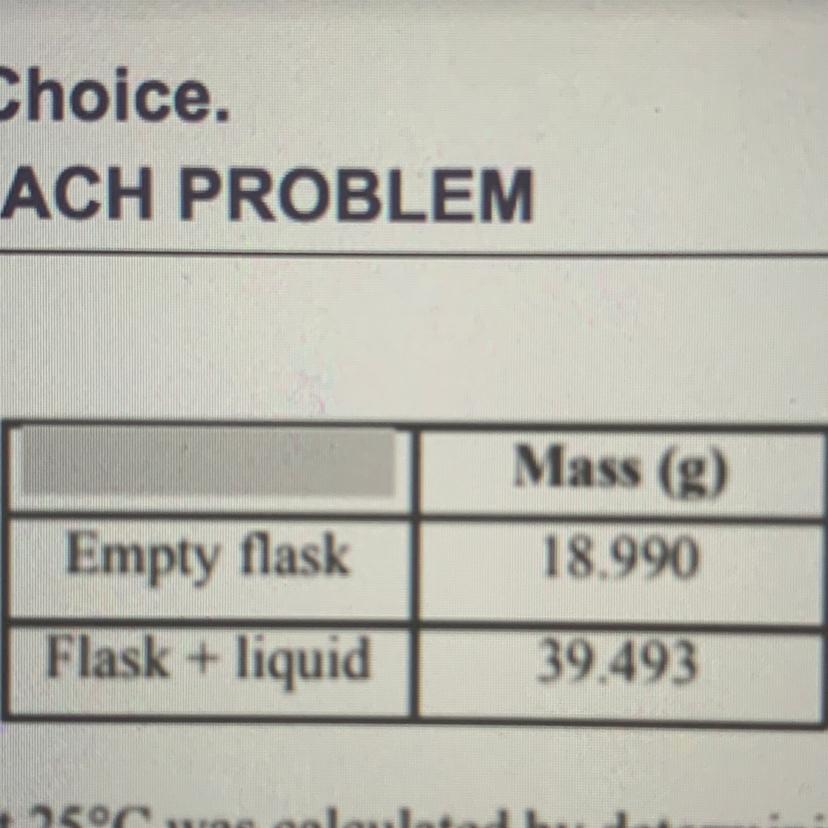
The density of a pure liquid at 25 degrees Celsius was calculated by determining the mass and volume of a sample of the liquid. A student measured the mass of a clean, dry 25.00 mL volumetric flask, filled the flask to its calibration mark with the liquid, and then measured the mass of the flask and liquid. The recorded measurements are shown in the table above. On the basis of this information, to how many significant figures should the density of the liquid be reported?
Answers
Answer
The density of the liquid is 0.82 g/mL.
Explanation:
From the question given above, the following data were obtained:
Mass of empty flask = 18.990 g
Mass of flask + liquid = 39.439 g
Volume of liquid = 25 mL
Density of liquid =..?
Next, we shall determine the mass of the liquid. This can be obtained as follow:
Mass of empty flask = 18.990 g
Mass of flask + liquid = 39.439 g
Mass of liquid =.?
Mass of liquid = (Mass of flask + liquid) – (Mass of empty flask)
Mass of liquid = 39.439 – 18.990
Mass of liquid = 20.503 g
Finally, we shall determine the density of the liquid as follow:
Mass of liquid = 20.503 g
Volume of liquid = 25 mL
Density of liquid =..?
Density = mass / volume
Density of liquid = 20.503 / 25
Density of liquid = 0.82 g/mL
Related Questions
At what temperature does water boil at 101kPa?
Answers
Answer:
100°C
Explanation:
A liquid boils when the vapour pressure is the same as the atmospheric pressure. At 101kPa or 1 atm, water boils at the well known number of 100°C. If the pressure is different, you will have to use the Clausius Clapeyron Equation.
Which is the control center for the endocrine system?
O the thymus
the hypothalamus
the pancreas
o the thyroid
Answer: the hypothalamus
Answers
Thank you for the answer, it is much appreciated, and if you haven't seen it already, it is B.
Answer:
The answer is B on edge
Explanation:
What is the wavelength of a radio wave with a frequency of 1.2 × 106 Hz? 3.6 × 1014 meters 3.6 × 102 meters 2.5 × 102 meters
Answers
Answer:
2.5 × 102 meters
Explanation:
Wavelength, which is denoted by λ, can be calculated using the formula:
λ = v/f
Where λ = wavelength (m)
v = speed of light (3×10^8m/s)
f = frequency (Hz)
According to the given information I n the question, f = 1.2 × 10^6 Hz, v = 3×10^8m/s
Hence;
λ = v/f
λ = 3 × 10^8 ÷ 1.2 × 106
λ = 3/1.2 × 10^ (8-6)
λ = 2.5 × 10^2
Therefore, the wavelength of the radio wave is 2.5 × 10^2 meters
A jogger with a mass of 60 kg is moving at 2 m/s. What is the jogger's kinetic energy?
Answers
Answer:
Ke = mgv
Explanation:
(60)(9.81)(2) =1177.2 joules persecond or Watts.
1.177 kilowatts
is muriatic acid a compound or not?
Answers
HCl + H2O ———-> H3O^+(aq) and Cl^-(aq)
Hydrochloric acid is a mixture of these ions
H2O and H3O^+(aq) and Cl^-(aq)
There are many different concentrations of hydrochloric acid - it must be a mixture.
What causes the air above a pot of boiling water to become warm?
The air transfers thermal energy to the water vapor.
The water vapor transfers thermal energy to the air.
The particles in the air lose kinetic energy.
The particles in the water vapor gain kinetic energy.
Answers
Answer: The water vapor transfers thermal energy to the air.
Explanation:
How do you think the amount of a material affects its tendency to sink or float?
ASAP
Answers
Answer:
it dependes on the material
Explanation:
what is the material
Answer:
it doesnt
Explanation:
The amount of material does not reflect on its tendency to sink or float because that depends on density which is an intensive property.
Which pair of symbols represents nuclei with the same number of neutrons?
A. 56 Co and 580
B. 57Co and 58Ni
c. 57Fe and 58 Ni
D. 57Mn and 57Fe
Answers
Answer:
B
Explanation:
Mass number = number of neutrons + number of protons
Atomic number is the number of protons in the nucleus.
58Ni : 58 is the number of neutrons + number of protons
atomic number of Ni is 28. Thus, 58 -28 = 30 neutrons
57Co ; 57 is the number of neutrons + number of protons
number of Co is 27. Thus, 57 – 27 = 30 neutrons
Molecules in a liquid are:
closer together than in a gas
moving more quickly than in a solid
moving more slowly than in a gas
all of the above
Please help!!!
Answers
Answer:
all of the above
Explanation:
All of the above
Explanation:
2. Calculate the density of a rock that has a mass of 21.58 grams and causes the water in a
graduated cylinder to rise from 20.0yl to 25.4 ml.
Answers
Answer:
4.00 g/mL
Explanation:
Density is mass divided by volume (g/mL).
Mass of rock = 21.58
Volume of rock = 25.4 - 20.0 = 5.4mL
Density of rock = 21.58g / 5.4mL = 3.99629 g/mL
Round to the lowest number of significant figures which is three = 4.00 g/mL
How r fossil fuel helpful
Plss help ill give brainliest
Answers
Answer: The United States gets 81% of its total energy from oil, coal, and natural gas, all of which are fossil fuels. We depend on those fuels to heat our homes, run our vehicles, power industry and manufacturing, and provide us with electricity.
Explanation:
....
Answer:
They give us things that we couldnt get anywhere else and arent harmful to the enviornment
Explanation:
Please help!!!
A piece of metal of mass 27 g at 93° C is placed
in a calorimeter containing 59.2 g of water at
21°C. The final temperature of the mixture is 34.9 ° C. What is the specific heat capacity of the metal? Assume that there is no energy lost to the surroundings.
Answer in units of J/g. ° C
Answers
Answer:
1.586 J/g°C
Explanation:
So, we have the formula [tex]q = mc\Delta t[/tex].
Since heat released by the metal is = to the heat absorbed by the water (because they eventually become the same temperature in solution), we can say
[tex]m_{water}C_{water}(T_{water}-T_f) = - m_{metal}C_{metal}(T_{metal} - T_f)[/tex]
Plugging in, we get:
[tex]59.2*4.184*(21- 34.9) = - 27*C_{metal}*(93 - 34.9)[/tex]
Solving, we get [tex]C_{metal} = 1.586[/tex] J/g°C.
the elements boron, silicon, germanium, and arsenic be classified into? *
Answers
Which term describes weathering?
breaks down rock
causes heating and cooling
makes pebbles and rocks larger
carries away fragments
Answers
Answer:
Breaks down rocks.
Explanation:
Weathering is the breaking down of rocks often with water. For example a sea shell will get batered with water and over time form sand.
Does the use of lithium cause human health problems?
Answers
Answer:
Yes
Explanation:
Lithium can cause nausea, diarrhea, dizziness, change in heart rhythm, muscle weakness, fatigue, and a dazed feeling
Continued use of Lithium could mean fine tremor, frequent urination, and thirst
Source: Webmd.com
a molecule for sulfur dioxide consists of one sulfur atoms (S) and two oxygen atoms (0). what is the chemical formula for this compound
Answers
Which landform is created by glaciers? A. sandbar B. moraine C. alluvial fan (will give brainliest to the best answer)
Answers
Answer: B: Moraine
Explanation:
Can someone help me with number two pls
Answers
Answer:
The answer is G
Explanation:
this is because of what a element looks like on a periodic table
Answer:
F. 1 and 3
Elements are pure substance
Arrange the following metals in order of increasing reactivity Pb,Al, Sn,Q,Au,and Mg.
Answers
Answer:
Sn, Q,Au, Al,Pb
Explanation:
Because thats hw that works
HOPE IT HElPS
Which among the following is/are correct about solution (true solution)?
I. Concentration of solute will always be the same throughout a mixture
in a solution.
II. Solutions do not show scattering of light.
(a) only I (b) only II
(c) both I and II (d) none
Answers
Answer
A
Explanation
letter a kase tama
with of the following describe a chemical change
a) a metal fork was melted
b) a metal fork was cut in half
c) a metal fork became bent
d) a metal fork became rusted
Answers
Answer:
D
Explanation:
Rusting is a chemical change that occurs due to oxygen.
Answer:
d i had this your welcome
When boiling a pot of water on the stove without a lid on the pot, the _____________. A energy and water molecules are free to escape into the surrounding environment. B molecules can escape out into the environment, but the energy are forced to stay in the system. C energy can escape out into the environment, but the molecules are forced to stay in the system. D both the energy and molecules are trapped in the system.
Answers
Answer:
A) Energy and Water molecules are free to escape into the surrounding environment.
Explanation:
Hello! This is the correct answer! (I took the K12 test)
I hope this helps! Have a blessed day! :)
When boiling a pot of water on the stove without a lid on the pot, the energy and water molecules are free to escape into the surrounding environment.
What is boiling?Boiling point of a substance is the temperature at which the substance changes from liquid state to gaseous state where these two systems comes in equilibrium.
Thus when a liquid is boiled, the liquid molecules get more energy to move apart and they escape into gaseous state. With change in state of the substance, the energy also be transferred from the liquid to the environment.
If the pot is closed with a lid, then both energy can be transferred but molecules get trapped inside. But here the pot is open and thus the gas molecules and energy are free to move outside.
Therefore, option A is correct for the boiling of water in an open pot.
To learn more about boiling, refer the link below;
https://brainly.com/question/23026747
#SPJ5
At 9°C a gas has a volume of 6.17 L. What is its volume when the gas is at standard temperature?
Answers
Answer:
V₂ = 5.97 L
Explanation:
Given data:
Initial temperature = 9°C (9+273 = 282 K)
Initial volume of gas = 6.17 L
Final volume of gas = ?
Final temperature = standard = 273 K
Solution:
Formula:
The Charles Law will be apply to solve the given problem.
According to this law, 'the volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure'
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 6.17 L × 273K / 282 k
V₂ = 1684.41 L.K / 282 K
V₂ = 5.97 L
2. What are the units for the mass of a solid? mass of a liquid?
Answers
Answer:
I'm not sure myself but I'd say mass since it's a solid
which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d8
Answers
Answer:
Darmstadtium
Explanation:
An element with the electronic configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s²5f¹⁴6d⁸ has 110 electrons in its electron shells.
Since the element is a neutral atom, this number is also equal to its atomic number. Therefore, its atomic number is 110.
The element in the period table that has an atomic number of 110 is Darmstadtium, a d-block element, thus a transittion metal. It also belong to period 7 in the Periodic table of elements.
The element that has the electron configuration is Darmstadtium.
Electronic configuration:Since An element with the electronic configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s²5f¹⁴6d⁸ contains 110 electrons in its electron shells.
Also, the element should be a neutral atom, this number should also equal to its atomic number. Therefore, its atomic number is 110.
The element in the periodic table that has an atomic number of 110 is Darmstadtium, a d-block element, thus a transition metal.
Learn more about electron here: https://brainly.com/question/24850343
Which of these is a physical change in shape?
A. Digesting food
B. Cooking an egg
C. Breaking a glass
D. Dissolving salt
Answers
2. When one plate is thrust under another plate it will create what landform?
a. Rift valley
b. Mountain range
C. Subduction zone-trench
d. Crater
Answers
Answer:
a
Explanation:
Need help ASAP please
Answers
Answer:Melting can create steam, kind of like a nukeular plant exept no nukulear rods
2. Exothermic; decreases
3. Endothermic;increases
4. Exothermic; decreases
5. Endothermic; increases
Which of the following is an example of quantitative data?
A.five eggs in each nest
B.oval-shaped eggs
C.small eggs
D.blue eggs with white specks
Answers
Explanation: quantitative means data that includes numbers
Which answer choice correctly describes what will happen to an element with four valence electrons if it engages in a chemical reaction?
a. It will lose electrons.
b. It will neither gain nor lose electrons.
c. It will gain electrons.
d. It may gain or lose electrons.