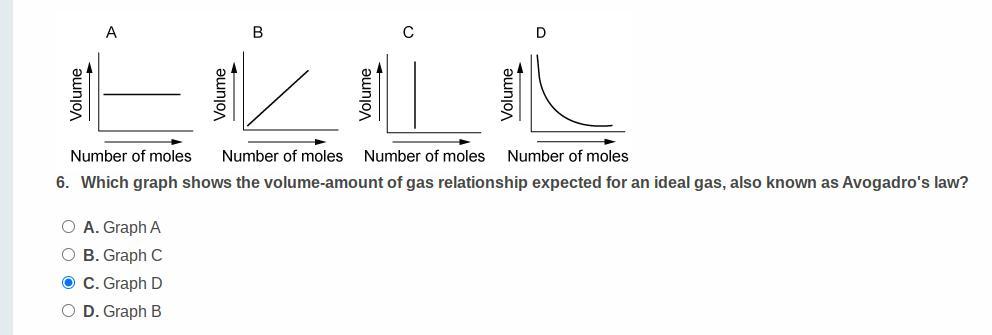
Answers
Answer:
The answer is A
Explanation:
I got it right on the test
Related Questions
Liquids B and C are partially miscible at 25 oC. When one starts with 1 mol of C at 25 oC and isothermally adds B a little at a time, a two-phase system first appears when a bit more than 0.125 mol of B has been added; continuing to add B, one finds the two-phase system becomes a one-phase system when a total of 3 mol of B has been added. For a system consisting of 2.5 mol of B and 2 mol of C at 25 oC, find the number of moles of B and of C present in each phase.
Answers
Answer:
[tex]0.8\overline 3[/tex] moles of A and 2.5 moles of B in the one-phase system
[tex]1.1 \overline 6[/tex] moles of A and [tex]0.1458 \overline 3[/tex] moles of B in the two-phase system
[tex]0.3541 \overline 6[/tex] moles of B remains in the system
Explanation:
The given parameters are;
The extent of miscibility of liquid B and C = Partially miscible
The number of moles of liquid B added to 1 mole of liquid A that forms a two-phase system = 0.125 mol of liquid B
The number of moles of liquid B added to 1 mole of liquid A that forms a one -phase system = 3 moles of liquid B
Whereby the system consist of 2.5 mol of B and 2 mol of C at 25°C, we have;
The 1 mole of A mixes with 3 moles of B to form a single phase solution
Therefore;
2.5 moles of B will mix with (1/3)×2.5 moles of A = 5/6 moles of A
The remaining number of moles of A in the system = 2 - 5/6 = 7/6 moles of A
Similarly, we have;
At least, 0.125 mole of B combines with 1 mole of A to form a two-phase system
7/6 moles of A will combine with 7/6 × 0.125 = 7/48 moles of B to form a two-phase system
The number of moles of B left = 0.5 - 7/48 = 17/48 = 0.3541[tex]\overline 6[/tex] moles of A
Therefore, we have;
5/6 moles of A and 2.5 moles of B in the one-phase system
7/6 moles of A and 7/48 moles of B in the two-phase system
17/48 moles of B remaining in the system
Which of these statements best supports the idea that a cell is the basic unit of a living organism?
A.
The number of cells in an organism affects the size of that organism.
B.
A tissue is composed of cells with similar structure and function.
C.
Some organisms have only one cell.
D.
All organisms are made up of one or more cells.
Answers
Answer:
The answer is D.
Explanation:
I searched it up :)
HELP WHOEVER ANSWERS WILL BE MARKED BRAINLIEST!!!
Answers
Balance the chemical equation
C2H6+O2 = CO2 +H2O
Answers
........................
A certain protein transports a sodium ion from the inside of the cell membrane to the outside using active transport. Which of the following must be true? A. There is no change in concentration before or after transport. B. The transport process did not use chemical energy. C. After transport, the concentration of protein in the cell decreases. D. After transport, the concentration of sodium is lower inside the cell.
Answers
Cool air can hold less water vapor than warm air. Apply this fact to explain why clouds and precipitation form on the windward side of the mountain.
Answers
The pressure is directly proportional to temperature (when the pressure decrease the temperature decrease too). Because the air parcel expands so the molecules will not interact with each other as much.
The energy of the particles does not change but the fact that the particles are more spaced out means the parcel is cooler.
so now, the warmer a parcel of air the more water vapor it can hold. so, if a parcel of air cools it's ability to hold water vapor drops and if it drops to a low enough point that is when the water vapor will condense and turn back into liquid water. This is how clouds and precipitation form on the the windward side of the mountain.
Clouds and precipitation form on the windward side of the mountain due to change in temperature and pressure.
What is the relation between pressure and temperature?
Temperature of any substance is directly proportional to the pressure of that substance.
If the temperature of the wind increases so that winds get warmer and at the same time pressure of gas also increases, due to which particles will go far from each other. After this warm air rises up and cools down will condenses to form clouds, after this precipitate will falls on the windward side of the mountain.
Hence due to temperature and pressure precipitate will form on the windward side of the mountain.
To know more about temperature & pressure, visit the below link:
https://brainly.com/question/25290815
#SPJ2
After you set up your apparatus for the second part of the experiment, your group begins to add the textbooks on top of the plunger. It appears that the plunger continues to drop with every added book and does not return to its original place after the books are removed. What possible sources of error could be given for this experimental outcome? Be specific and give at least two reasons.
Answers
The possible sources of error that could be given for this experimental outcome is the change in the density of the air.
What is an experiment?An experiment simply means the procedure that is carried out in order to refute a particular hypothesis. It should be noted that experiments are important to provide insights into the cause and effect of a particular situation.
In this situation, with every added book, the volume of that air column will reduce. It is just important that the temperature should remain constant when the gas compression is taking place.
Another source of error is the assumption of moles that was assumed was constant during the experiment. In this scenario, it's possible that some gas could have escaped while the compression was taking place.
Learn more about experiments on:
brainly.com/question/17274244
#SPJ1
The extraction of iron from ore is represented by the chemical reaction equation.
Fe2O3 + 3CO → 2Fe + 3CO2
Calculate the mass of carbon (II) oxide required for the reduction of 40 kg of iron (III) oxide
Answers
Answer:
21 Kg of CO.
Explanation:
The balanced equation for the reaction is given below:
Fe₂O₃ + 3CO —> 2Fe + 3CO₂
Next, we shall determine the masses of Fe₂O₃ and CO that reacted from the balanced equation. This can be obtained as illustrated below:
Molar mass of Fe₂O₃ = (56×2) + (16×3)
= 112 + 48
= 160 g/mol
Mass of Fe₂O₃ from the balanced equation = 1 × 160 = 160 g
Molar mass of CO = 12 + 16 = 28 g/mol
Mass of CO from the balanced equation = 3 × 28 = 84 g
SUMMARY:
From the balanced equation above,
160 g Fe₂O₃ required 84 g of CO.
Finally, we shall determine the mass of of CO required for the reduction of 40 kg of Fe₂O₃. This can be obtained as follow:
From the balanced equation above,
160 g Fe₂O₃ required 84 g of CO.
Therefore, 40 Kg of Fe₂O₃ will require = (40 kg × 84 g) / 160 g = 21 Kg of CO.
Thus, 21 Kg of CO is required for the reaction.
Copper has a specific heat of 0,900 J(g C). How much energy in kJ is needed to raise the temperature of a 700 g block of aluminum from 30.7°C to 82,1C?(show your steps)
Answers
Answer:
32.4 kJ
Explanation:
Given that:
Specific heat capacity fo copper, c = 0.9
Mass of copper, m = 700 g
Temperature change, dθ ;t2 - t1 = (82.1 - 30.7) = 51.4
Energy required, E = mcdθ
E = 700 * 0.9 * 51.4
E = 32382 J
Energy required on kJ
32382 / 1000
= 32.382 kJ
= 32.4 kJ
Which of the following is a characteristic of both the Earth and the Moon?
Fossils and fossil
fuels under the
surface
Rocky surface
covered with
landforms
Flowing water in
Oceans and rivers
Atmosphere
containing oxygen
Answers
Answer:
rocky surface covered with landforms
Explanation:
(b) The student collects the H2(g) produced by the reaction and measures its volume over water at 298 K after carefully
equalizing the water levels inside and outside the gas-collection tube, as shown in the diagram below. The volume is
measured to be 45.6 mL. The atmospheric pressure in the lab is measured as 765 torr, and the equilibrium vapor pressure
of water at 298 K is 24 torr,
45
46
Gas
Water
Calculate the following.
() The number of moles of h2 produced in the reaction
Answers
Answer:
The pressure of H₂(g) = 741 torr
Explanation:
Given that:
The atmospheric pressure measured in the lab = 765 torr
The vapor pressure of water = 24 torr
By applying Dalton's Law of Partial Pressure :
Making The Pressure inside the tube due to the H₂(g) the subject of the formula :
we have:
= (765 -24) torr
= 741 torr
According to ideal gas law and Dalton's law of partial pressure, 0.0136 moles of hydrogen are produced in the reaction.
What is ideal gas law?The ideal gas law is a equation which is applicable in a hypothetical state of an ideal gas.It is a combination of Boyle's law, Charle's law,Avogadro's law and Gay-Lussac's law . It is given as, PV=nRT where R= gas constant whose value is 8.314.The law has several limitations.It is applicable to ideal gases which have hypothetical existence.Law was proposed by Benoit Paul Emile Clapeyron.
In the given problem, according to Dalton's law of partial pressure, pressure =765-24=741 torr
Substituting the given values in the ideal gas equation, n=PV/RT
n=741×45.6×10[tex]^-3[/tex]/8.314×298=0.0136 moles.
Thus 0.0136 moles of hydrogen are produced in the reaction.
Learn more about ideal gas law,here:
https://brainly.com/question/28257995
#SPJ5
Plasma can be found naturally in
O water and ice.
O stars and lightning.
O wood and metal.
O comets and asteroids.
Answers
Answer:
stars and lightning. this is the answer ok
Hope it helped
What is the mass of 0.063x10^-4 moles of aluminum sulphate ?
Answers
Answer:
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.
Explanation:
Aluminum sulfate Al₂(SO₄)₃ has a molar mass of 342.15 g/mol.
Molar mass is the amount of mass that a substance contains in one mole of a substance, which can be an element or a compound.
So in this case you can apply the following rule of three: if 342.15 grams are present in 1 mole of aluminum sulfate, how much mass is present in 0.063*10⁻⁴ moles of the compound?
[tex]mass of aluminum sulphate=\frac{0.063*10^{-4}moles*342.15 grams }{1 mole}[/tex]
mass of aluminum sulphate= 2.15*10⁻³ grams
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.
How many grams equal 4.3 x 1024 atoms of oxygen (02)?
Answers
Answer: 248.66g
Explanation:
4.3e24 / 6.23e23 = 6.9 mols O2
6.9(18.02*2)=248.66
Rick is creating a love potion for Morty. To make the potion, Rick's needs 51 mL of a mixture solution where 40% is carbonated water. After checking around his shop, Rick finds two solutions he could use. The first solution he found is 65% green tea, 15% carbonated water, and 20% whole milk. The second solution is 17% orange juice, 38% lemonade, and 45% carbonated water. How much of the first solution and second solution does Rick need to mix together to create the love potion? Round your final answers to one decimal place. You may solve this problem using any method we have learned in the class.
Answers
Answer:
The amount of the first solution rick needs to mix together to create the love portion is 8.5 mL
Explanation:
So as to make the love potion, we have;
The percentage of carbonated water in the love portion = 40%
The percentage of green tea in the first solution = 65%
The percentage of carbonated water in the first solution = 15%
The percentage of whole milk in the first solution = 20%
The percentage of orange juice in the second solution = 17%
The percentage of lemonade in the second solution = 38%
The percentage of carbonated water in the second solution = 45%
Let 'x' represent the volume in mL of the first solution added to make the love portion, and let 'y' be the volume in mL of the second solution added to make the love portion, we have;
x + y = 51...(1)
0.15·x + 0.45·y = 0.40×51 = 20.4
0.15·x + 0.45·y = 20.4...(2)
Solving the system of simultaneous equation by making 'y' the subject of each of the equation gives;
For equation (1)
y = 51 - x
For equation (2)
y = 20.4/0.45 - (0.15/0.45)·x = 136 - 3·x
y = 136/3 - (1/3)·x
Equating the two equations of 'y', gives;
51 - x = 136/3 - (1/3)·x
51 - 136/3 = x - (1/3)·x
17/3 = (2/3)·x
(2/3)·x = 17/3
x = (3/2) × (17/3) = 17/2 = 8.5
x = 8.5
y = 51 - x = 42.5
y = 42.5
Therefore, the amount of the first solution rick needs to mix together to create the love portion, x = 8.5 mL
I need help with like 35 questions anyone willing to help please let me know I have discord
UnknownGoddxss#2795
Please I only have like 5 hours to complete this
Answers
Answer and Explanation:
The molar mass of a substance is all of the weights from the elements combined.
So, we have the elements
CU, S, and [tex]O_{4}[/tex]
CU has a mass is 65
S mass is 32
O has a mass of 16, but there's 4 atoms of O, so we do 16 times 4, which is 64.
Now we add.
65 + 32 + 64 is 161.
So, the answer is 160, or answer choice A.
#teamtrees #PAW (Plant And Water)
Can u please help me name which one it is
Answers
sorry if it’s wrong
Answer:
I think it is C
Explanation:
A 497.9-gallon steel storage tank contains 91.7kg of methane. If the temperature is 23.7˚C what is the pressure inside the tank?
Answers
Answer:
74.08 atm
Explanation:
We'll begin by converting 91.7 kg to grams (g). This can be obtained as follow:
1 kg = 1000 g
Therefore,
91.7 kg = 91.7 kg × 1000 g / 1 kg
91.7 kg = 91700 g
Next, we shall determine the number of mole present in 91700 g of methane, CH₄. This can be obtained as follow:
Mass of CH₄ = 91700 g
Molar mass of CH₄ = 12 + (4"1)
= 12 + 4
= 16 g/mol
Mole of CH₄ =?
Mole = mass /Molar mass
Mole of CH₄ = 91700 / 16
Mole of CH₄ = 5731.25 moles
Next, we shall convert 497.9 gallon to litres (L). This can be obtained as follow:
1 gallon = 3.785 L
Therefore,
497.9 gallon = 497.9 × 3.785
497.9 gallon = 1884.55 L
Next, we shall convert 23.7 ˚C to Kelvin temperature. This can be obtained as follow:
T(K) = T(˚C) + 273
T(˚C) = 23.7 ˚C
T(K) = 23.7 °C + 273
T(K) = 296.7 K
Finally, we shall determine the pressure in the tank as follow:
Number of mole (n) = 5731.25
Volume = 1884.55 L
Temperature (T) = 296.7 K
Gas constant (R) = 0.0821 atm.L/Kmol
Pressure (P) =?
PV = nRT
P × 1884.55 = 5731.25 × 0.0821 × 296.7
P × 1884.55 = 139607.92
Divide both side by 1884.55
P = 139607.92 / 1884.55
P = 74.08 atm
Therefore the pressure in the tank is 74.08 atm
How many atoms are in 1.50 moles of Zinc?
Answers
Kevin is working on a model that shows the positions of Earth, the Moon, and the Sun during the phases of
the Moon. How should he position them to show a New Moon?
Answers
Answer:
Explanation:what’s the answer
Answer: A. With Earth between the Moon and the Sun.
Explanation:
Which set of coefficients will correctly balance the following skeleton equation?
Fe + Cl2 →FeCl3
Answers
Answer:
2Fe + 3Cl2 → 2FeCl3
Explanation:
What is the atomic mass of element XX if 3.6 moles of the substance has a mass of 192 grams?
Answers
Answer:
53.3g/mol
Explanation
Moles=mass/molar mass
Molar mass= mass/moles
Molar mass= 192/3.6
= 53.3
Pls someone help rn!! Pls pls
Answers
Answer:
A. Mass = 61kg, weight on moon = 97.8N
Explanation:
To calculate the weight (force) from Mass of a body, the following formula is used:
W = mg
Where;
W = weight (Newtons)
m = mass (kg)
g = acceleration due to gravity (9.8m/s²)
According to this question, a scale shows that I weigh 600N on Earth, the mass on Earth can be calculated thus:
Using W = mg
m = W/g
m = 600/9.8
m = 61.2
m = 61kg
However, this mass on Earth will weigh differently on the Moon because gravity in the moon is 1.6 N/kg. The weight of the 61kg on the Moon is:
W(moon) = mass × gravity
W = 61 × 1.6
W = 97.6 N
Therefore, Mass = 61kg, weight on moon = 97.8N
Astronauts go to live on the International Space Station for a period of ________ month(s). A one B three C six D nine
Answers
Answer:
The answer is C. six months
Explanation:
I hope this helps you bestie:))
Question 2 (1 point)
(02.01 LC)
Which scientist proposed the model of an atom as a solid sphere? (1 point)
John Dalton
Niels Bohr
Ernest Rutherford
William Crookes
Answers
Answer:
John Dalton
Explanation:
Both John Dalton and Democritus thought that the atom was an indivisible sphere until J.J. Thompson came out with the plum pudding model. Hope I helped!
Sulfur
how many electron shells in 4th shell?
Answers
Answer:
16 electrons in a sulfur atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and six in shell three.
Explanation:
Fill in the blanks. 3NH3
Answers
Answer:
3, 9, 3
Explanation:
The coefficient of 3 tells us that there are three molecules (the chemical unit of NH3). Each molecule of ammonia (NH3) is made up of 1 atom of nitrogen bonded to 3 atoms of hydrogen.
Since there are three molecules, we have three times the amount of atoms there are in one molecule.
3 x 1 = 3 nitrogen
3 x 3 = 9 hydrogen
chemical properties of elements
Answers
Answer:
Atomic number. The atomic number indicates the number of protons within the core of an atom. ...
Atomic mass. The name indicates the mass of an atom, expressed in atomic mass units (amu). ...
Electronegativity according to Pauling. let me know if you need more ;)
Elizabeth REALLY didn’t study, and she also wrote the formula for dicarbon tetrafluoride as “C2F5”. Describe why her answer is incorrect.
Answers
Answer:
correct formula: C2F4
Explanation:
tetra means 4. There are 2 C and 4 F in dicarbon tetrafluoride.
The subscript represents the number of ____________ in a chemical equation.
Answers
Good luck!