Answers
Answer:
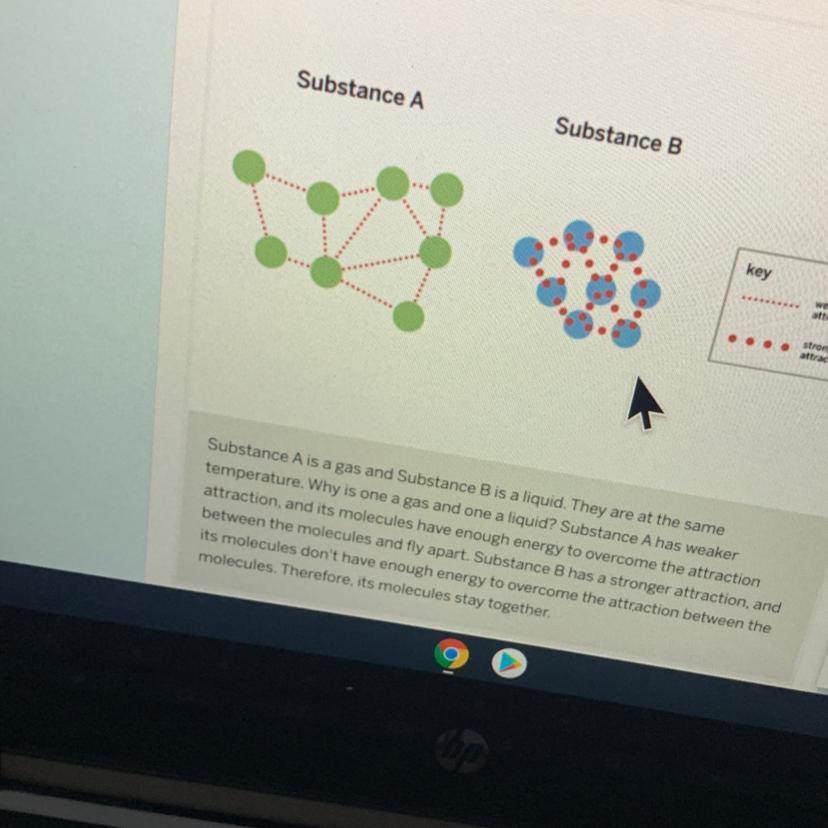
I think it would be a liquid.
Explanation:
If the temperature hits a certain point, it will turn to ice. If the ice gets exposed to heat, it will melt and return back to it's natural form, a lqiuid. If the liquid is exposed to enough heat, it will evaporate. Not sure if this is right, but I tried my best!
Related Questions
2.50 L of gas originally at 50 K is warmed to 80 K. If the pressure remains constant, which gas law needs to be used to find the new volume?
Select one:
a.Charles law
b.Gay-Lussac’s law
c.Boyle’s law
d.Combined gas law
Answers
Which statement best describes DNA?
(A) It can be found in every living cell.
B It can be found only in specialized
cells.
© It can be found only in cells with a
nucleus.
D It can be found only when cells are
dividing.
Answers
Answer:
Found in every living cell
explain the laws of universal graviation?
Answers
Answer:
Newton's law of universal gravitation is usually stated as that every particle attracts every other particle in the universe with a force that is directly proportional to the product of their masses and inversely proportional to the square of the distance between their centers.
Can Intrusive and Extrusive Igneous rocks form from the same mineral composition?
Answers
EXAMPLE took the test k12
Determine the percent yield for the reaction between 46.5 g of
ZnS and 13.3 g of oxygen if 18.l4 g of ZnO is recovered along with an unknown quantity of sulfur
dioxide.
please show work thank you
Answers
The percentage yield of the reaction has been 46.74%.
Percentage yield can be given as the ratio of the actual yield to the theoretical yield.
For the theoretical yield, the balanced equation will be:
[tex]\rm 2\;ZnS\;+\;3\;O_2\;\rightarrow\;2\;ZnO\;+\;2\;SO_2[/tex]
Thus, 2 moles of ZnS will give 2 moles of ZnO.
The moles of 46.5 g ZnS:
Moles = [tex]\rm \dfrac{weight}{molecular\;weight}[/tex]
ZnS = [tex]\rm \dfrac{46.5}{97.474}[/tex]
ZnS = 0.477 mol
The moles of 13.3 grams Oxygen:
Oxygen = [tex]\rm \dfrac{13.3}{32}[/tex]
Oxygen = 0.415 mol.
The moles of 18.14 grams ZnO:
ZnO = [tex]\rm \dfrac{18.14}{81.38}[/tex]
ZnO = 0.222 mol
According to the balanced chemical equation,
2 moles ZnO = 2 moles ZnS
0.222 mol ZnO = 0.222 mol ZnS
2 moles ZnO = 3 moles Oxygen
0.222 mol ZnO = 0.334 mol of Oxygen
Since, both the reactants are in enough concentration,
The theoretical yield can be calculated with any of the reactants. The theoretical yield of ZnO from 46.5 grams of ZnS can be given as:
1 mole ZnS = 1 mol ZnO
0.477 mol of ZnS = 0.477 mol of ZnO.
The mass of 0.477 mol of ZnO:
Mass = moles [tex]\times[/tex] molecular weight
Mass of ZnO = 0.477 [tex]\times[/tex] 81.38
Mass of ZnO = 38.818 grams.
The theoretical yield of ZnO = 38.818 g.
The actual yield of ZnO = 18.14 g.
Percentage yield = [tex]\rm \dfrac{18.14}{38.81}\;\times\;100[/tex]
Percentage yield = 46.74 %
The percentage yield of the reaction has been 46.74%.
For more information about the percentage yield, refer to the link:
https://brainly.com/question/11715808
The kinetic molecular theory, explains how particles in matter behave.
True
False
Answers
Answer:
true
Explanation:it is hard to explan
A pebble is dropped into a cup of water and sinks to the bottom ofthe cup. A solid metal bead of exactly the same size is dropped into the same cup and sinks to the bottom of the cup. How do the pebble and the metal bead compare?
Answers
Answer:
A solid metal bead of the same size is dropped into the same cup and sinks to the bottom of the cup. How do the pebble and the metal bead compare? They both have the same mass. They are both denser than water.
Explanation:
nun
Answer:
Both the pebble and metal bead of the same mass will be equally accelerated if they are at the same place, in this case, they are in the same place meaning they will be accelerated at the same speed towards the earth. Thus even if they are in the water they will sink at the same speed and finally will reach the bottom of the cup.
The formula for zirconium(VI) dichromate
Answers
Answer:
Zr(Cr2O7)2
Explanation:
Name the following compound:
CH2 ≡ CH2
Ethyl
Ethyne
Ethane
ethane
Answers
Answer:
Answer is A. Ethyl.........
Answer:
D. ethane
Explanation:
Please someone help me ...............
Answers
Answer:
Q58. number of moles(n) and relative formula mass(Mr)
Q59. a) 2 H2S + O2 --> 2 H2O + 2 S
b) After the balanced equation you should able to draw it yourself - I cannot draw it on here :)
Explanation:
A reaction rate is the change in of a reactant or product with
Answers
Answer:
the rate of reaction is the speed at which a chemical reaction takes place
What happens when dilute sulphuric acid is poured on a copper plate?
Answers
NH₄NO₃ → N₂O + 2H₂O When 45.70 g of NH₄NO₃ decomposes, what mass of each product is formed?
Answers
Answer: 25.13 g of [tex]N_2O[/tex] and 20.56 g of [tex]H_2O[/tex] will be produced from 45.70 g of [tex]NH_4NO_3[/tex]
Explanation:
To calculate the moles :
[tex]\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}[/tex]
[tex]\text{Moles of} NH_4NO_3=\frac{45.70g}{80.04g/mol}=0.571moles[/tex]
The balanced chemical equation is:
[tex]NH_4NO_3\rightarrow N_2O+2H_2O[/tex]
According to stoichiometry :
1 mole of [tex]NH_4NO_3[/tex] produce = 1 mole of [tex]N_2O[/tex]
Thus 0.571 moles of [tex]NH_4NO_3[/tex] will require=[tex]\frac{1}{1}\times 0.571=0.571moles[/tex] of [tex]N_2O[/tex]
Mass of [tex]N_2O=moles\times {\text {Molar mass}}=0.571moles\times 44.01g/mol=25.13g[/tex]
1 mole of [tex]NH_4NO_3[/tex] produce = 2 moles of [tex]H_2O[/tex]
Thus 0.571 moles of [tex]NH_4NO_3[/tex] will require=[tex]\frac{2}{1}\times 0.571=1.142moles[/tex] of [tex]H_2O[/tex]
Mass of [tex]H_2O=moles\times {\text {Molar mass}}=1.142moles\times 18g/mol=20.56g[/tex]
Thus 25.13 g of [tex]N_2O[/tex] and 20.56 g of [tex]H_2O[/tex] will be produced from 45.70 g of [tex]NH_4NO_3[/tex]
If 120 g of potassium chloride are mixed with 100 g of water at 80°C, how much will not dissolve?
Answers
Answer:
yes
Explanation:
How many grams of KCl will dissolve in 1 liter of H2O at 50 °C? 5. 58.0 g of K2Cr2O7 is added to 100 g H2O at. 0 °C. With constant stirring, to what temp-.
Describe a time in the group discussion when you referred to the notes you took, the graphic organizer you filled out, and your position paper.
Answers
Answer:
To back up any statements I make.
To refute any errors presented.
Explanation:
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
What is a group discussion?The term group discussion has to do with learners talking about a topic in a manner ion which each learner takes a turn to explain a portion of the topic.
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
Learn more about group discussion:https://brainly.com/question/11940982
#SPJ2
Please help not to fail chemistry
Answers
Answer:
I think the heat on the surface or the fire under the dish cost evaporation coz when the water got heat tge water evaporate or go to the upper part of dish and cool down then back to the bottom.
Explanation:
Plss correct me if im wrong
In the following reaction 2Na+ 2H20 - 2NaOH + H2: all of the sodium is consumed and a small amount of
liquid water remains at the end of the reaction. Which reactant is the limiting reagent?
A) H20
B) H2
C) Na
D) NaOH
Answers
Answer:
C) Na
Explanation:
The question tells us that all of the sodium (Na) is consumed and that water is left over. This means that sodium would be the limiting reagent.
I took the test
The force that is pulling down on a person or object on the surface of the Earth is
called (attraction of matter to matter)?
normal force
mass
weight
gravity
Answers
Have a good day/night
what kind of chemical reaction is this?
Answers
Plz help! I will give brainliest.
Answers
Answer:
D. 0.50
Explanation:
Use avogadro number to find the whole work.
C1 Progress quiz: Atomic structure 2 - test
4 The atomic radius of a silicon atom is 1.1 x 10-10 m. Calculate its atomic radius in
nanometres.
O
11 nm
o
0.011 nm
0.11 nm
1.1 nm
Answers
Answer:
0.11 nm
Explanation:
1.1 x 10-10 m
The goal is to convert the atomic radius from meters (m) to nanometres (nm). We do this by multiplying the value by 10^9.
This is given as;
[tex]1.1 * 10^{-10} * 10^{9}\\1.1 * 10^{-10 + 9}\\1.1 * 10^{-1}\\0.11[/tex]
The Earth has how many satellites?
(i know this is not chemistry theres no science one)
Answers
Answer:
Well it depends, There are THOUSANDS of inactive satellites. Even scientists don't know how many as for active ones there is about 1 957 according to nasa
Explanation:
How many particles are in one mole of copper (II) sulfate, CuSO4?
Answers
What is the pOH if the [OH-]= 0.165 M? What is the pH of this basic solution? *Please round your answer(s) to the appropriate number of significant figures. Your answer can be in standard notation or i letter "e" in place of x10.* 1 N
Answers
Answer:
78
Explanation:
Answer:
The OH would be .7825 and the basic solution is a strong base.
Explanation:
What you would do is -log(0.165) in your calculator which would give you 0.7825160065 as an answer. Im not sure what the significant figure is so I will not be rounding to that, but that is your answer for the first part.
The second part: because your pOH is a .7825, this would be consiered a strong base in the pOH because it is closer to 1 which is your base.
What is an example of quantity of the resource being used by humans that affected
Answers
Answer:
1. Healthy and well educated people contribute to their societies in positive way
2. Malnourished and illiterate people cannot contribute much to their respective societies.
Explanation:
Molar Volume of a gas at STP=22.4 L Example 3: Determine the volume of Carbon dioxide created when 15.0 grams of NaHCO3 are decomposed into water, Sodium carbonate, and Carbon dioxide at STP?
Answers
Answer:
20009 is answer is right 1345678899444
Explanation:
thhjbfthvcdthvctyhgffdy
gvhjk ghkde ghjcddxxb hhj hhgddxb ggjbcxdss ggbbsrtyg fy gbsdgh
PLEASE HELP ME QUICKLY!
Imagine you are at your favorite beach. The sun is shining and you are enjoying the ocean breeze. The temperature is about 89F. You take your shoes off and realize that the sand is almost too hot to walk on. You run to the water's edge to wet your feet after the walk over the sand and realize that the water is almost too cold. Explain the temperature difference between the sand and water using Thermodynamics.
Answers
Answer:
The specific heat of water is more specific than the heat of sand, therefore, it will take more energy to raise the same amount of water with the same temperature.
Explanation:
The temp is 89 F so, 31.67° C
The specific heat of the water is 4.184 J/g° C
The specific heat of the sand is 0.290 J/ g° C
The heat is the amount of energy that is needed to raise the temp. of the substance
You will need a lot more energy to raise the temp of the water then of the same amount of sand, therefore, because of the specific lower heat of the sand it will raise it's temperature quicker compared to water.
is butanoic acid a hydrocarbon?
Answers
Yes butanoic acid is a hydrocarbon.
what is computer hardware in short?
Answers
Answer:
computer hardware is a collection of a physical part of a computer system
examples = CPU , monitor , mouse etc
The diagram shows a pedigree for a recessive inherited trait. If H represents the dominant allele and h represents the recessive allele what are the genptypes of the parents shown in the pedigree.
Answers
Answer:
Hh
Explanation:
if you have a dominant allele and a recessive allele they'd be different (heterozygous)
If H represents the dominant allele and h represents the recessive allele, Hh is the genotypes of the parents shown in the pedigree.
What is genotypes?The type of variation present at a specific locus (i.e., region) in the genome is scored by what is known as a genotype. Symbols can be used to symbolize it. For illustration, BB, Bb, and bb might be employed to denote a certain gene variant.
The actual DNA sequence, such as CC, CT, or TT, can also serve as a representation of a genotype. In a single experiment, the genotypes spanning millions of sites in a genome can be determined using DNA sequencing as well as other techniques. If H represents the dominant allele and h represents the recessive allele, Hh is the genotypes of the parents shown in the pedigree.
Therefore, Hh is the genotypes of the parents shown in the pedigree.
To know more about genotypes, here:
https://brainly.com/question/16882362
#SPJ5