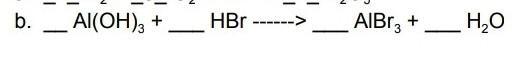Answers
Answer:
The answers are 1:3:13
Explanation:
when I had to do these types of equations just search them up on you-tube lol theres this guy called Wayne Breslyn that does a really good job of explaining and has like the answers for any and everyy balancing equation
Related Questions
what is the meaning of flourish?
Answers
*For Example* :
"wild plants flourish on the banks of the lake"
Rubidium (Rb) has two isotopes. Using the data in the table below,
calculate the relative atomic mass of Rb. Give your answer to 3 significant
figures.
Answers
Answer:
85.6
Explanation:
(72 x 85) + (28 x 87)
-----------------------------
100
= 85.56
= 85.6 (3 s.f.)
how many molecules of NaCl are in 32.5 G
Answers
Answer :32,5 g NaCl equal 0,556 moles.
Explanation:
Q3 - Complete the word equation *
1 point
Bromine +
Chlorine
Bromide +
Potassium
Chloride
Bromine +
Potassium
Chloride
Hydrogen
bromide +
Potassium
Chloride
Chlorine +
Potassium
Bromide ->
Answers
how do fossils get inside rocks
Answers
Explanation:
It to be fossilized, the remains usually to be covered by sediment soon after death. sediment can include the sandy seafloor,lava,and even sticky tar. Over time, minerals in the sediment seep in to remains. The remains become fossilized.
A 2.00 g sample of ammonia (NH3) reacts with 4.00 g of oxygen (O2) according to the equation 4NH3+5O2→4NO+6H2O. How much excess reactant remains after the reaction has stopped ( knowing that NH3 is the excess reactant )?
Answers
Answer:
0.017 moles of ammonia remains after the reaction is stopped.
Explanation:
The reaction is:
4NH₃ + 5O₂ → 4NO + 6H₂O
The first step is to convert the mass to moles, of each reactant:
2 g . 1mol/ 17g = 0.117 moles of NH₃
4 g . 1mol /32g = 0.125 moles of O₂
Ammonia, states the question, is the excess reactant so we can confirm it,
5 moles of oxygen need 4 moles of ammonia to react (by stoichiometry)
Then, 0.125 moles of oxygen may react to (0.125 . 4) / 5 = 0.1 moles
As we have 0.117 moles of ammonia and we need 0.1 moles.
(0.117 - 0.1) = 0.017 moles remains after the reaction is completed.
If we convert the moles to mass we have:
0.017 mol . 17 g /1mol = 0.289 g
-->
Give the products of this neutralisation reaction: Sulfuric acid + magnesium hydroxide
Answers
Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas.
similarities of ancient and modern philosophy
Answers
Name the following cycloalkane:
CH2CH2CH2CH3
A. butylcyclohexane
B. butylcyclopentane
C. propylcyclohexane
Answers
Answer:
The answer for this question is A. butylcyclohexane
Explanation:
Choose letter A
You welcome have a nice day.
Name of the given compound is butyl cyclohexane.
What are cyclo alkanes?Cyclo alkanes are those organic compounds in which alkanes are present in the cyclic form.
In the nomenclature of cyclo alkane, name of the substitute is put before the name of the main ring. In the given structure number of carbons in ring is 6 so that given cycloalkane is cyclohexane and butyl group is attached as substitute. So name of the given compound is butyl cyclohexane.
Butyl cyclopentane is wrong because 6 carbons are present in ring.Propylcyclohexane is also wrong because 4 carbons are attached in the ring.Hence name of the compound is butyl cyclohexane.
To know more about cycloalkanes, visit the below link:
https://brainly.com/question/25058711
#SPJ2
the indicated gene codes for a protein made up of the amino acid
Answers
Answer:
N/A
Explanation:
Is this a true or false answer or.....?
1. If you have 5g of pennies how many dozen pennies do you have?
Answers
Answer:
15.69 dozen
Explanation:
Mass of penny = 5 g
Dozens of penny =..?
Next, we shall convert 5 g to gross. This can be obtained as follow:
3824 g = 1000 gross
Therefore,
5 g = 5 g × 1000 gross / 3824 g
5 g = 1.3075 gross
Thus, 5 g is equivalent to 1.3075 gross.
Finally, we convert 1.3075 gross to dozen. This can be obtained as follow:
1 gross = 12 dozen
Therefore,
1.3075 gross = 1.3075 gross × 12 dozen / 1 gross
1.3075 gross = 15.69 dozen
Thus, 5 g of penny is equivalent to 15.69 dozen
Round the following number to
3 significant figures:
3,545,530
Answers
The concept of significant figures are mainly used by scientist and engineer to know the significance of digits in a measurement. Therefore, 3,545,530 upto 3 significant figures is 3.54 x 10³.
What is significant figures?Significant figures are the figures that indicate the degree of accuracy of a value. It tells about the precision of a value. It gives an idea about the digits that are necessary to indicate the experimental value.
Rules for counting significant figures are:
Number between 1 to 9 is always significant
Zeroes after a number has got no significance
Zeroes before a number has got no significance
Zeroes between number has got significance
3,545,530 upto 3 significant figures is 3.54 x 10³.
Therefore, 3,545,530 upto 3 significant figures is 3.54 x 10³.
To learn more about Significant figures, here:
https://brainly.com/question/12656148?
#SPJ1
Please help me with dis
Answers
Answer:
c
Explanation:
Measurements show that unknown compound X has the following composition:
element mass %
carbon 74.8%
hydrogen 25.1%
Write the empirical chemical formula of X. please help
Answers
the empirical chemical formula of X is CH4
Explanation:Step 1: Imagine you have a sample of compound weighing exactly . Multiply the mass of this sample by the mass percents to find the mass of each element in the sample
Step 2: Divide the mass of each element by the element's molar mass to find the moles of each element in the sample. Remember to round your answers to the correct number of significant digits.
Step 3: Divide the moles of each element by the the smallest number of moles of any element to find the mole ratio of elements in the sample.
Step 4: Multiply the mole ratio by the smallest whole number that changes it into a whole number ratio to find the atom ratio of elements in the sample.
Note that the result of each multiplication must equal a whole number only within measurement uncertainty.
The measurement uncertainty in this calculation comes from the measurement uncertainty of the mass percents given in the question. The mass percents each have significant digits. That means each mass percent has some measurement uncertainty in the third significant digit, and only the first two significant digits can be considered completely reliable.
In Step 1 you multiplied each mass percent by something with zero uncertainty (the exactly you assumed your sample weighed), in Step 2 you divided by a measurement with more than significant digits (the molar mass of the elements), and in Step 3 you divided by a measurement with the same number of significant digits (the least number of moles of any element in the compound). None of these steps added to the measurement uncertainty of your calculation.
Therefore, the measurement uncertainty in the final result of all your calculations is determined by the measurement uncertainty in the original mass percents and will be in the third significant digit. That means the result of each of the final multiplication steps must equal a whole number only to within the first two significant digits.
The whole numbers in the last column of the table are and .
6. The Haber process for making ammonia (NH)
gas from its elements was developed by Fritz Haber
during WWI. Haber hoped to use ammonia as
fertilizer to grow food for Germany during the
Allie's blockade. How many liters of hydrogen
would be required to produce 40.0L of ammonia at
STP? N+H, NH,
Answers
Answer:
60 Liters
Explanation:
The equation for this reaction is given as;
N2 + 3H2 → 2NH3
From the reaction;
3 mol of H2 produces 2 mol of NH3
At STP;
1 mol = 22.4 L
This means
67.2 L ( 3 * 22.4) of H2 produces 44.8 L ( 2 * 22.4) of NH3
How many L of H2 would produce 40 L of NH3
67.2 = 44.8
x = 40
Solving for x;
x = 40 * 67.2 / 44.8
x = 60 L
How are chemical reactions classified from an energy point of view?
Answers
Answer:
normanie tykohoukdh kjdj jj jjd
Explanation:
Can anyone help me with this algebraic expression
(4a+3b)² (3a-4b)²
Answers
Answer:
=144a⁴−168a³b−239a²b²+168ab³+144b⁴
Explanation:
=144a⁴−168a³b−239a²b²+168ab³+144b⁴
Answer:
[tex](4a + 3b)(4a + 3b)(3a - 4b)(3a - 4b) \\ {16a}^{2} + 12ab + {9b}^{2} + 12ab + {9a}^{2} + 12ab + 12ab + {16b}^{2} \\ {16a}^{2} + {9a}^{2} + {9b}^{2} + {16b}^{2} + 12ab + 12ab + 12ab + 12ab \\ {25a}^{2} + {25b}^{2} + 48ab[/tex]
A balloon occupies 175 cm at 25.0 C. What volume will it occupy at 42.5 °C?
Answers
175 cm³ instead of 175 cm.
Answer:
297.5 cm³
Explanation:
From the question;
Initial volume; V1 = 175 cm³
Initial temperature; T1 = 25°C
Final temperature; T2 = 42.5°C
From Charles law we can find the volume V2 from the equation;
V1/T1 = V2/T2
Making V2 the subject gives;
V2 = V1 × T2/T1
V2 = (175 × 42.5)/25
V2 = 297.5 cm³
What is the percent composition of each element in the compound below:
CrPO4
Answers
HELP PLEASE
What does the 2 mean in the formula of calcium nitrate? What does the 3
mean?
Answers
Answer:
Calcium nitrate is an inorganic compound with the chemical formula Ca(NO3)2. ... It is a nitrate salt of Calcium which contains calcium and nitrogen and oxygen. Calcium nitrate is a white or whitish-grey coloured granular solid which absorbs moisture from the air and is usually found as a tetrahydrate compound Ca(NO3)2.
Explanation:
A gas in the form of Bubbles is released is it a chemical change or a physical change
Answers
Answer:
The formation of a gas is a clue to chemical changes. The bubbles of gas that you observed form when an antacid is dropped into water is an example of change. Another clue that a chemical change has occurred is the formation of a solid.
Explanation:
please help! Will give Brainliest!
Answers
Answer: the answer is c
Explanation:
also, is that canvas? sajdasds
How does pressure affect an organism plants?
Answers
Answer:
Turgor pressure in plants. Turgor pressure within cells is regulated by osmosis and this also causes the cell wall to expand during growth.
Explanation:
I hope that helps
What are the reactants in the following equation; NaOH + HCI --> NaCl + H2O
a)NaOH and HCI
b)HCI and H20
c) NaOH and NaCl
d)HCI and water
Answers
What are the charges on ions of Group 1A, Group 3A (aluminum), and Group 5A?
Answers
Answer:
Group 1A: 1+
Group 3A: 3+
Group 5A: 3+ or 5+
Explanation:
PLEASE HELP MEEE THIS IS FOR a gradE AND IF I FAIL I FaIL THIS CLASs HELP ME
Answers
Answer:
stream
Explanation:
because a stream goes into a lake
Answer:
D) Stream
Explanation:
If combining cation A with a +3 charge and anion X with a -3 charge, how would we write the compound?
O AX
O A3X3
O 3AX
Answers
Answer: it’s not A3X3
Explanation:
1. AX
For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion.If a cation A carries +3 charge and an anion X carries -3 charge, the compound so formed is AX. Since the charges gets cancelled out.For example: consider aluminum phosphate, aluminum carries +3 charge and phosphate carries -3 charge, the molecular formula for the compound will be AlPO₄.Learn more:
brainly.com/question/11164440
Which date has the longest daylight and the shortest darkness
Answers
The Winter Solstice.
How many grams are in 8.50 e 23 formula units of NaCl?
a)82.5 g
b)8.25 g
c)85.0 g
d)91.5 g
Answers
Answer:
a)82.5 g
Explanation:
To solve this problem we will use Avogadro's number, which states the number of formula units per mole.
8.50x10²³formula units ÷ 6.023x10²³ formula units/mol = 1.41 molThen we convert 1.41 moles of NaCl to grams, using its molar mass:
1.41 mol NaCl * 58.44 g/mol = 82.4 gThe closest answer is option a).
PLEASE HELP ASAP!!! WILL MARK BRAINLIEST!!!! 25 POINTS!!!!! IF YOU"RE JUST GOING TO ANSWER FOR POINTS PLEASE DO NOT I NEED REAL ANSWERS!!!!
2. Which gas law is this experiment investigating? How does your graph represent the gas law under investigation?
3. Using your knowledge of the kinetic molecular theory of gases, describe the relationship between volume and temperature of an ideal gas. Explain how this is reflected in your lab data.
4. Pressure and number of moles remained constant during this experiment. If you wanted to test one of these variables in a future experiment, how would you use your knowledge of gas laws to set up the investigation?
Answers
Answer:
2. for number two I asks what the experiment is showing, I need the picture of the experiment.
3. As temperature increases, the particles will gain kinetic energy causing it to move more rapidly and randomly. However, this causes the gas to expand as the particles will have more energy to roam freely. as temperature increases, Volume increases.
4. Im not sure D: