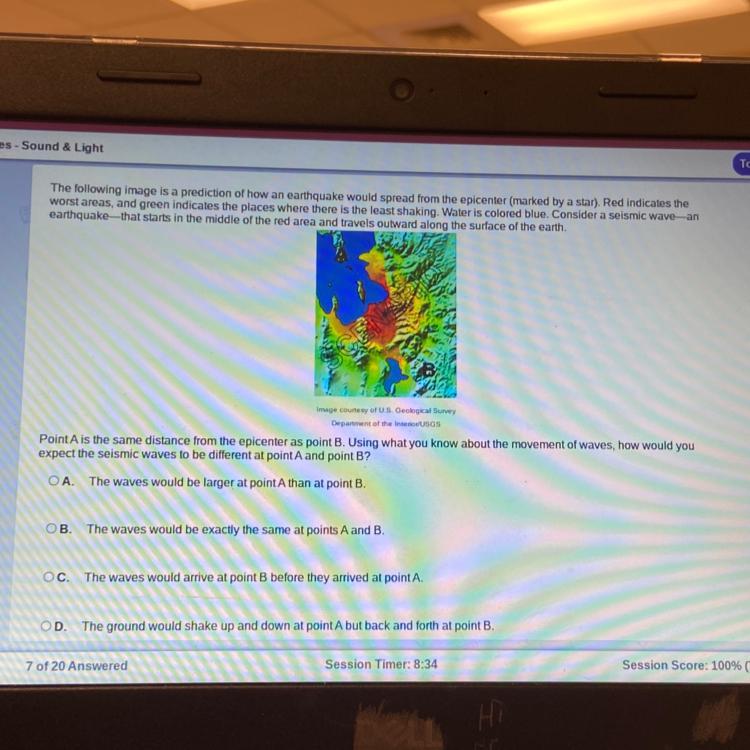
The following image is a prediction of how an earthquake would spread from the epicenter (marked by a star). Red indicates the
worst areas, and green indicates the places where there is the least shaking. Water is colored blue. Consider a seismic wave-an
earthquake that starts in the middle of the red area and travels outward along the surface of the earth.
Image courtesy of U.S. Geological Survey
Department of the Interio/
USOS
Point A is the same distance from the epicenter as point B. Using what you know about the movement of waves, how would you
expect the seismic waves to be different at point A and point B?
OA.
The waves would be larger at point A than at point B.
OB. The waves would be exactly the same at points A and B.
OC. The waves would arrive at point B before they arrived at point A.
OD
The ground would shake up and down at point A but back and forth at point B.
Answers
Answer:
its B
Explanation:
The earthquakes are occurring more in the land region of earth surface. Hence, the waves would arrive at point B before they arrived at point.
What is an earthquake?When two layers of the ground abruptly slide past one another, an earthquake results. The fault or fault plane is the area where they slide. The epicenter is the point on the earth's surface that is directly above the hypocenter, which is where the earthquake begins under the surface.
The energy that would typically force the blocks to move past one another is being saved up while the fault edges are glued together and the rest of the block is moving.
All that accumulated energy is released when the force of the sliding blocks ultimately displaces the resistance of the sharp edges of the fault and causes it to unstick. Like ripples on a pond, the energy radiates from the fracture in all directions as seismic waves. Therefore, point B in the land surface will first experience the seismic waves.
To find more on earthquakes, refer here:
https://brainly.com/question/29500066
#SPJ2
Related Questions
HELP PLEASE I NEED HELP THANKS I LOVE U
How many moles of potassium nitrate (KNO3) are there in 0.300 L of a 2 molar solution?
Answers
Answer:
0.500-Molarity solution
Explanation:
The moles of the compound is given as the number of atomic mass unit in the compound. The moles of potassium nitrate in the solution are 0.6 mol
What is molarity?The molarity is the concentration unit, and it can be defined as the moles of compound present in the liter of solution.
The molarity can be expressed as:
[tex]\rm Molarity=\dfrac{Moles}{Volume(L)}[/tex]
The given potassium nitrate solution has, molarity = 2 M
The volume of the solution is 0.3 L.
Substituting the values for the moles of the compound:
[tex]\rm 2\;M=\dfrac{Moles}{0.3\;L} \\\\Moles=2\;\times\;0.3\;mol\\Moles=0.6\;mol[/tex]
The moles of potassium nitrate in 2 M solution is 0.6 mol.
Learn more about moles, here:
https://brainly.com/question/15209553
You are given 127 g H2CO3 (Carbonic Acid) and a volume of 800 mL of water, what will the final concentration of your solution be? (The final unit is the Molar (M), but do not include it.) (Round to 3 decimal places.)
Answers
Answer:
2.562
Explanation:
First we convert 127 g of H₂CO₃ to moles, using its molar mass:
127 g ÷ 62 g/mol = 2.05 mol H₂CO₃Then we convert 800 mL to L:
800 mL / 1000 = 0.800 LFinally we can calculate the concentration of the solution as molarity:
molarity = moles / litersmolarity = 2.05 mol / 0.800 L = 2.562 MWhat does Ra Ra Ah Ah Ah Ro Ma Ro Ma Ma Ga Ga O La La mean?
Answers
Answer:
They are symbols of elements.
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Answer:
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Explanation:
They are elements
Help pls ASAP thank you!!
Answers
Answer:
Deer, Eagle, Raccoon, frog, turtle, fish, dragonfly.
Explanation:
They interact from the life cycle they eat each other...
How many sulfur atoms are in 3.7 mol of SO2?
× 10 S atoms
Answers
Answer: 22.3 *10^23 S atoms
Explanation:
2) Calculate the percent composition of each element in Mgso,
3) Calculate the percentage of each element in Ag,o.
Answers
Answer:
2)
[tex]\% Mg=20.2\%\\\\\% S=26.6\%\\\\\% O=53.2\%[/tex]
3)
[tex]\% Ag=93.1\%\\\\\% O=6.9\%[/tex]
Explanation:
Hello!
2) In this case, since magnesium sulfate is MgSO₄, we can see how magnesium weights 24.305 g/mol, sulfur 32.06 g/mol and oxygen 64.00 g/mol as there is one atom of magnesium as well as sulfur but four oxygen atoms for a total of g/mol; thus the percent compositions are:
[tex]\% Mg=\frac{24.305}{120.36 } *100\%=20.2\%\\\\\% S=\frac{32.06}{120.36 } *100\%=26.6\%\\\\\% O=\frac{64.00}{120.36 } *100\%=53.2\%[/tex]
3) In this case, although the element seems to contain Ag and O, we infer its molecular formula is Ag₂O; thus, since we have two silver atoms weighing 215.74 g/mol and one oxygen atom weighing 16.00 g/mol for a total of 231.74 g/mol, we obtain the following percent compositions:
[tex]\% Ag=\frac{215.74}{231.74} *100\%=93.1\%\\\\\% O=\frac{16.00}{231.74} *100\%=6.9\%[/tex]
Best regards!
In a single replacement reaction, the chloride ion in NaCl can be replaced
by
a. potassium
b. carbon
c.fluorine
d.neon
Answers
Answer:
c. fluorine
Explanation:
A single replacement reaction is defined as the chemical reaction in which a strong molecule replaces the weak molecule from a compound.
In a single replacement reaction, the chloride ion in NaCl can be replaced by fluorine and gives Sodium fluoride as fluorine (F) is stronger than chlorine (Cl) and from other given molecules also. So the single replacement reaction between NaCl and F will be:
NaCl + F2 => NaF + Cl2
Hence, the correct answer is "c. fluorine".
How many moles of hydrogen are in a 200. mg tablet of naproxen sodium, C14H13NaO3, the active ingredient in Aleve? Please show your work to receive full credit. MM = 252
Answers
Answer:
0.0103 mol
Explanation:
Step 1: Given data
Mass of the naproxen sodium tablet (m): 200. mgMolar mass of naproxen sodium (M): 252 g/molStep 2: Convert "m" to grams
We will use the conversion factor 1 g = 1000 mg.
200. mg × 1 g/1000 mg = 0.200 g
Step 3: Calculate the moles (n) of naproxen sodium
We will use the following expression.
n = m/M = 0.200 g / (252 g/mol) = 7.94 × 10⁻⁴ mol
Step 4: Calculate the moles of hydrogen in 7.94 × 10⁻⁴ moles of naproxen sodium
According to the chemical formula, the molar ratio of naproxen sodium to H is 1:13. The moles of H are 13/1 × 7.94 × 10⁻⁴ mol = 0.0103 mol.
Gravitational potential energy depends on the
Answers
Answer:
Gravitational potential energy depends on basically two factor;
Object’s position Mass of objectExplanation:
The distances between both the bodies, as well as the mass within each object, are factors that influence gravitational potential energy.
What is the IUPAC name for the following compound?
CH3-CH2-CH2-CH2-CH2-CH2 -CH2-CH3
Enter the name of the molecule.
Answers
Can someone please help me!!
Answers
Answer:
Less in the top
your well wisher
Methods of Heat Transfer
Identify the following examples of heat transfer as conduction, convection, or radiation.
1. Touching a metal spoon that is sitting in a pot of boiling water.
Will mark brainliest
Answers
Answer:
1
Explanation:
touching a metal spoon ....................water.
PLEASE HELP ME! I beg you :(
Answers
Hydrogen can be produced according to the following word equation
=zinc +hydrochloric acid =zinc chloride +hydrogen [zn^+2]
Write a complete balanced chemical equation for this chemical reaction?
Answers
Answer:
Zn + 2HCl → ZnCl₂ + H₂
Explanation:
First we write the equation using the molecular formulas instead of words:
Zn + HCl → ZnCl₂ + H₂We know zinc chloride is ZnCl₂ as the problem tells us the oxidation state of zinc in the products is +2, and chloride means Cl⁻¹.
Now we proceed to balance the reaction:
There are 2 Cl atoms and 2 H atoms on the right side, so we add a coefficient of 2 to HCl on the left side:
Zn + 2HCl → ZnCl₂ + H₂All forms of energy can be traced back to
Answers
Please help me thanks so much
Answers
Answer:
a switch
Explanation:
Answer:
a switch is the answer hope it helps
Please please help me please please help please please help me please please help please please help me
Answers
Answer:
AU9NJ-BLVHV-TCLJS-54YTB
find the number of molecules in 35.20 g of nitrogen dioxide
Answers
Answer:
4.584*10^23 molecules
Explanation:
Find molar mass of nitrogen and oxygen on periodic table.
find molar mass of nitrogen dioxide
use this to find moles of nitrogen dioxide
multiply by avagadros number
Suppose you want to make an acetic acid/acetate buffer to a pH of 5.00 using 10.0 mL of 1.00 M acetic acid solution. How many milliliters of 1.00 M sodium acetate solution would you need to add? The pKa for acetate buffer is 4.75.
Answers
Answer:
Explanation:
Molarity of NaOAc needed
Using the Henderson-Hasselbalch Equation calculate base molarity needed given [HOAc] = 1.00M and pKa(NaOAc) = 4.75 and [HOAc] = 1.00m.
pH = pKa + log [NaOAc]/[HOAc]
5.00 = 4.75 + log[NaOAc]/[1.00M]
5.00 - 4.75 = log [NaOAc] - log[1.00M]
log [NaOAc] = 0.25 => [NaOAc] = 10⁰·²⁵ M = 1.78
Given 10ml of HOAc, how much (ml) 1.78M NaOAc to obtain a buffer pH of 5.00.
Determine Volume of Base Needed
(M·V)acid = (M·V)base => V(base) = (M·V)acid / (M)base
Vol (NaOAc) needed = (1.00M)(0.010L)/(1.78M) = 0.0056 liter = 5.6 ml.
Checking Results:
5.00 = 4.75 + log [1.78M]/[1.00M] = 4.75 + 0.25 = 5.00 QED.
The volume of 1.00 M sodium acetate solution needed to prepare an acetic/acetate buffer of pH 5.00 using 10.0 mL of 1.00M acetic acid solution is 17.8 mL.
We can find the volume of the acetate solution with the Henderson-Hasselbalch equation:
[tex]pH = pka + log(\frac{[CH_{3}COONa]}{[CH_{3}COOH]})[/tex] (1)
Where:
[CH₃COOH] = 1.00 M
[CH₃COONa] =?
pH = 5.00
pKa = 4.75
From equation (1), we have:
[tex] log(\frac{[CH_{3}COONa]}{[CH_{3}COOH]}) = pH - pKa [/tex]
[tex] \frac{[CH_{3}COONa]}{[CH_{3}COOH]} = 10^{pH - pKa} [/tex]
[tex] \frac{[CH_{3}COONa]}{[CH_{3}COOH]} = 10^{5.00 - 4.75} = 1.78 [/tex]
Now, the volume of the acetate solution is:
[tex]\frac{n_{CH_{3}COONa}/Vt}{n_{CH_{3}COOH}/Vt} = 1.78[/tex]
Since the total volume is the same, we have:
[tex]\frac{n_{CH_{3}COONa}}{n_{CH_{3}COOH}} = 1.78[/tex]
[tex] \frac{[CH_{3}COONa]_{i}*V_{b}}{[CH_{3}COOH]_{i}*Va} = 1.78 [/tex]
Solving for Vb
[tex] Vb = \frac{1.78*[CH_{3}COOH]_{i}*Va}{[CH_{3}COONa]_{i}} = \frac{1.78*1.00M*10.0mL}{1.00 M} = 17.8 mL [/tex]
Therefore, we need to add 17.8 mL of sodium acetate solution.
Find more here:
https://brainly.com/question/24188850?referrer=searchResults
I hope it helps you!
What is the enthalpy change(Q) when 60.0 g of NaOH is dissolved in one litre of water, given that the temperature of the solution increased by 15.8 °C?
Answers
Answer:
ΔH = -44.029 KJ/mol
Explanation:
We are told that 60.0 g of NaOH is dissolved in one litre of water.
From conversion, 1 litre = 1000 g
From tables, specific heat capacity of water; c = 4.18 J/g/°C
We are given ΔT = 15.8 °C
Formula for the heat absorbed by the solution is given as;
Q = mcΔT
Where;
Q = Heat gained by the water
m = Mass of the water
c = Specific heat of water
ΔT = change in temperature
Thus;
Q = 1000 × 4.18 × 15.8
Q = 66044 J
The solution absorbed 66044 J and therefore it means that the dissolution of the salt gave off 66044 J.
Thus;
Enthalpy is; ΔH = -66044 J
Now, Mass of NaOH is 60 g.
We know that molar Mass of NaOH is 39.997 g/mol. Thus;
Converting to moles, we have;
n = 60/39.997
n = 1.5 mol
Now, enthalpy when 1.5 moles of NaOH are dissolved in water will be;
ΔH = -66044 J/1.5 moles
ΔH = -44029.33 J/mol
ΔH = -44.029 KJ/mol
How do the causes of surface and deep water currents differ?
A. Surface currents are caused by wind deep water currents are caused by difference in water density
B. Surface currents are caused by the Coriolis effect deep water currents are caused by differences in water density
C. Surface currents are caused by differences in water salinity deep water currents are caused by differences in water temperature
D. Surface currents are caused by differences in water density deep water currents are caused by wind
Answers
Answer:
The answer is A. Surface currents are caused by wind deep water currents are caused by difference in water density.
How much does the Earth weigh?
Answers
5.972 × 10^24 kg
hope it helps
Answer:
5.972 × 10^24 kg
hope this helps
What is N for ClO3, the
chlorate ion?
Answers
Answer:
Explanation: There is no molecule or ion as ClO3. The correct formula of chlorate ion is ClO3-. bolivianouft and 4 more users found this answer helpful.
Explanation:
As the [h+] in a solution decreases, what happens to the [OH^-]?
А. It increases and the pH increases.
B. It increases and the pH decreases.
C. It increases and the pH stays constant.
D. It decreases and the pH increases.
E. It decreases and the pH decreases.
Answers
Explanation:
as the Hydrogen (acid) ion decreases there is a corresponding increase in the Hydroxide (basic) ion.
Help me with my homework plzy
Answers
Answer:
the second answer choice i think
Explanation:
Five different substances added breakers of water. Each substance begins as a white, solid powder. Which substances do not dissolve in water? Salt,sugar,starch,chalk, and bakeing powder
Answers
Answer:
Chalk is does not dissolve in water
Explanation:
i had the same question as you
"A 4.75-kg cell phone is dropped from your second floor balcony (from rest). It hits the
ground at a speed of 40 m/s. Assuming air resistance can be ignored, calculate the
gravitational potential energy of the cell phone before it was dropped."
Answers
What is the mole fraction of KCI in a
mixture of 0.564 g NaCl, 1.52 g KCI,
and 0.857 g LiCl?
Molar Mass
NaCl: 58.44 g/mol
KCI: 74.55 g/mol
Lici: 42.39 g/mol
Answers
Answer:
Mole fraction of KCl = 0.4056
Explanation:
We'll begin by calculating the number of mole of each compound. This can be obtained as follow:
For NaCl:
Mass NaCl = 0.564 g
Molar mass of NaCl = 58.44 g/mol
Mole of NaCl =?
Mole = mass /Molar mass
Mole of NaCl = 0.564 / 58.44
Mole of NaCl = 0.0097 mole
For KCl:
Mass KCl = 1.52 g
Molar mass of KCl = 74.55 g/mol
Mole of KCl =?
Mole = mass /Molar mass
Mole of KCl = 1.52 / 74.55
Mole of KCl = 0.0204 mole
For LiCl:
Mass LiCl = 0.857 g
Molar mass of LiCl = 42.39 g/mol
Mole of LiCl =?
Mole = mass /Molar mass
Mole of LiCl = 0.857 / 42.39
Mole of LiCl = 0.0202 mole
Next, we shall determine the total mole in the mixture. This can be obtained as follow:
Mole of NaCl = 0.0097 mole
Mole of KCl = 0.0204 mole
Mole of LiCl = 0.0202 mole
Total mole =?
Total mole = Mole of NaCl + Mole of KCl + Mole of LiCl
Total mole = 0.0097 + 0.0204 + 0.0202
Total mole = 0.0503 mole
Finally, we shall determine the mole fraction of KCl in the mixture. This can be obtained as follow:
Mole of KCl = 0.0204 mole
Total mole = 0.0503 mole
Mole fraction of KCl =?
Mole fraction of KCl = Mole of KCl /Total mole
Mole fraction of KCl = 0.0204 / 0.0503
Mole fraction of KCl = 0.4056
Answer:
.4 is correct
Explanation:
Joan wants to test if salt lowers the temperature at which water boils. In two or more complete sentences, describe the best way for Joan to develop a hypothesis for this situation. Write your answer in the essay box below.
Answers
Answer:
Joan's hypothesis should make a prediction about the answer to the question. A hypothesis is just an educated guess on what you think the outcome will be on the experiment. The prediction must be testable and stated in if-then form. For example, a good hypothesis for Joan's experiment could be, I think that 1/2 cup of salt will make the water boil quicker, than water without salt.
Explanation:
Hope it helped!
Help plz now !
Which statement explains why a chemical equation must be balanced?
A. It must show the reactants and products on the correct sides of
the reaction arrow.
B. It must show that the mass of each element involved is conserved
by a chemical reaction.
C. It must show how each chemical formula is written to accurately
represent each substance.
D. It must show that coefficients and subscripts can be used in
chemical formulas.
Answers
Answer: B. It must show that the mass of each element involved is conserved by a chemical reaction.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The correct statement why a chemical equation must be balanced is It must show that the mass of each element involved is conserved by a chemical reaction.
Answer:
B. It must show that the mass of each element involved is conserved
by a chemical reaction.
Explanation:
a p e x, just took the quiz