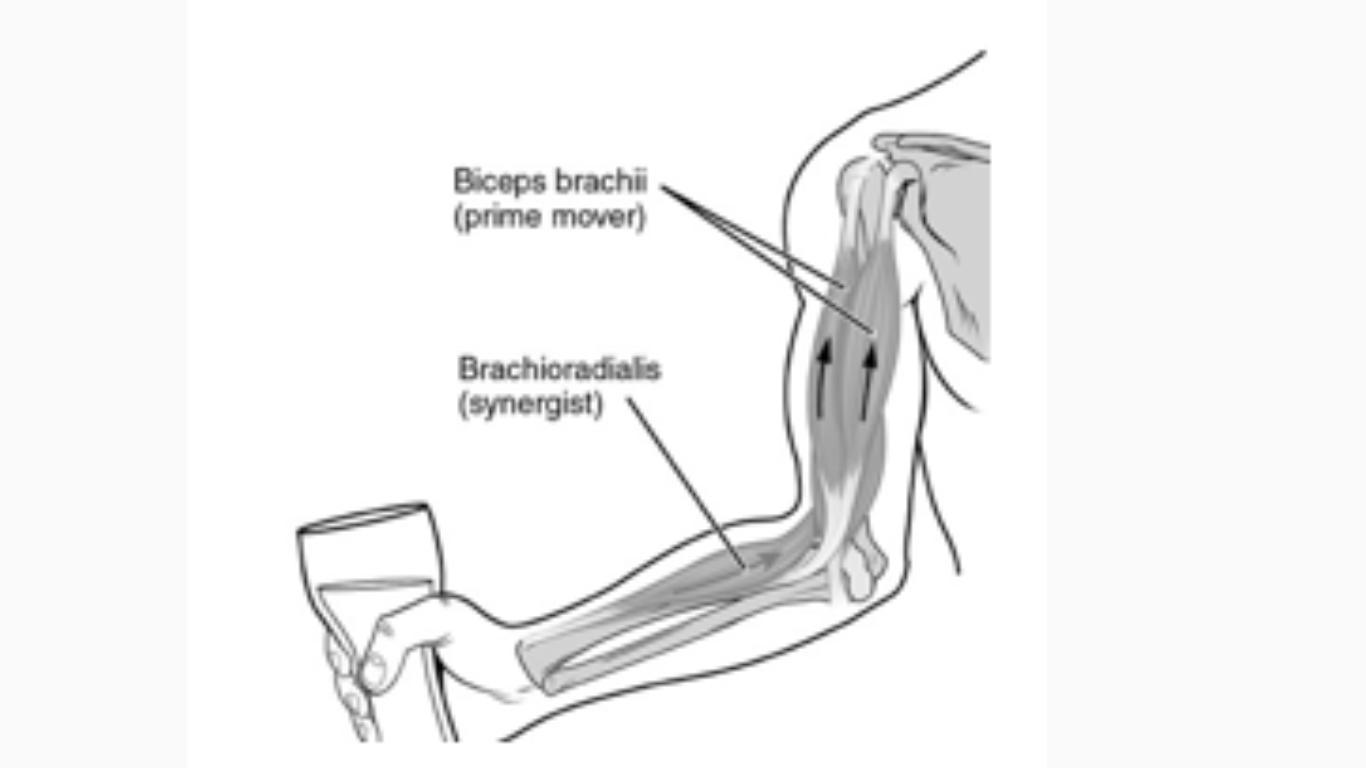
The model below shows a part of a human body's muscular system.
Muscles are organs that make movement possible. Which organ system is responsible for controlling muscular movement?
A. Endocrine system
B. Skeletal system
C. Nervous system
D. Digestive system
Answers
Answer:
you must go to jones im doing the same test
Explanation:
I did the quiz
Related Questions
What would you see if you were standing on the Earth's surface in the penumbra and you were looking up at the sky?
Answers
Answer:
clouds?
Explanation:
im sorry i just wanted the 23 points
how many molecules of water are found in 1.5mol of water ?
Answers
Answer:
9.033×10^23 Molecules
Explanation:
We are given,
No.of moles of water = 1.5 moles
The approximate value of Avagadro's Constant =6.022×10^23 particles
Hence,
As we know that,
No.of particles = No.of moles × Avagadro's Constant
Here,
No. Of Water Molecules in 1.5 moles of water = 1.5×6.022×10^23
No.of Water Molecules in 1.5 molesof water=9.033×10^23 Molecules
Can someone help me
Answers
Answer:
The longer it takes to orbit the sun.
Explanation:
22
Silicon(IV) oxide is a covalently bonded compound.
Which statements are correct?
1
2
Silicon atoms form four single bonds in silicon(IV) oxide.
Oxygen atoms form two double bonds in silicon(IV) oxide.
Silicon(IV) oxide has a high melting point.
Silicon(IV) oxide contains one silicon atom and four oxygen atoms.
3
4
с
D
2 and 3 only
3 and 4 only
B
1 and 3 only
1 and 2 only
А
А
Answers
Answer:
2 and 3 only
Explanation:
The formular for the compound; Silicon(IV)oxide is SO2
1. Silicon atoms form four single bonds in silicon(IV) oxide.
This statement is wrong because the silicon is doubly bonded to two oxygen atoms.
2. Oxygen atoms form two double bonds in silicon(IV) oxide.
This statement is correct.
3. Silicon(IV) oxide has a high melting point.
This statement is correct Due to it's macromolecular structure, it is able to form thousands of covalent bonds between it's sulphur and oxygen sub units.
4. Silicon(IV) oxide contains one silicon atom and four oxygen atoms.
This statement is wrong as silicon(iv) oxide contains only two oxygen atoms.
Mike mixes two chemicals in a container. The container quickly becomes
very hot
What can be said about this reaction?
Answers
Answer:
it is an exothermic reaction
1.Identify the types of bonds between the carbon and the two oxogen molecules in the carbon dioxide molecule
2.then describe the bond angle
3. Explain how the structural formula can be used to determine bond angles .
Answers
2A bond angle is the angle between two bonds originating from the same atom in a covalent species
3. 1.Write the Lewis dot structure for the molecule.
2.Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule.
3. Use the VSEPR shape to determine the angles between the electron domains.
Stop telling people how you feel they do not wanna hear you're problems tell them to God man these people don't care what you gotta say I just had to tell y'all this and write it in a notebook please I'm tryna tell y'all that's the key kings and queens stay humble 2021 is the new year and new u
Answers
Answer:
Preach!
Explanation:
Can someone answer this for me?
Answers
Answer: The moles of nitrogen dioxide produced are 96.3
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number [tex]6.023\times 10^{23}[/tex] of particles.
To calculate the moles, we use the equation:
[tex]\text{Number of moles}=\frac{\text{Given volume}}{\text {Molar volume}}=\frac{3714L}{22.4L}=165.8moles[/tex]
[tex]4NH_3(g)+7O_2(g)\rightarrow 4NO_2(g)+6H_2O(g)[/tex]
According to stoichiometry:
7 moles of oxygen [tex](O_2)[/tex] produce = 4 moles of nitrogen dioxide [tex](NO_2)[/tex]
Thus 168.5 moles of oxygen [tex](O_2)[/tex] will produce = [tex]\frac{4}{7}\times 168.5=96.3[/tex] moles of nitrogen dioxide [tex](NO_2)[/tex]
If there are 5 protons, how many electrons does the atom have?
Answers
If there are 5 protons, there should be 5 electrons as well. Boron as an electron configuration of 2-3 or 1s2 2s2 2p.
What is proton ?The term proton is defined as a subatomic particle found in the nucleus of every atom. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron.
The protons are considered as elementary particles. The protons are called to be composite particles, containing three valence quarks, and together with neutrons are now classified as hadrons.
Boron has an atomic number of 5. In which it has 5 protons. Therefore, the atom also has 5 electrons. Its atomic mass is 10.811. Thus, it has 11 total particles in its nucleus i.e., protons and neutrons.
Thus,If there are 5 protons, there should be 5 electrons as well. Boron as an electron configuration of 2-3 or 1s2 2s2 2p.
To learn more about the proton, follow the link;
https://brainly.com/question/1252435
#SPJ2
Help me plz I don’t know what it’s mean
Answers
Answer:
1 has the highest density because it has the most amount of circles in the least amount of space- it is the most densely filled with circles; it is the most dense.
Answer:
image 1 would have the highest density
Explanation:
the closer the molecules are the more solid an object is. if the molecules are far apart and rarely bump into eachother, it is more gaseous. the object in the middle (2) is a liquid
5) Determine the mass if lithium hydroxide that is produced when 12.87 g of lithium nitride reacts with
an excess of water according to the following process:
Li,N + 3 H2O + 3 LiOH + NH,
Answers
Answer:
Mass = 26.58 g
Explanation:
Given data:
Mass of lithium nitride = 12.87 g
Mass of lithium hydroxide produces = ?
Solution:
Chemical equation:
Li₃N + 3H₂O → 3LiOH + NH₃
Number of moles of of Li₃N:
Number of moles = mass/molar mass
Number of moles = 12.87 g/ 34.83 /mol
Number of moles = 0.37 mol
now we will compare the moles of lithium nitride with lithium hydroxide.
Li₃N : LiOH
1 : 3
0.37 : 3/1×0.37 = 1.11 mol
Mass of LiOH:
Mass = number of moles × molar mass
Mass = 1.11 mol × 23.95 g/mol
Mass = 26.58 g
PLS HELP ASAP I DONT KNOW WHAT TO WRITE AND I ONLY HAVE 6 MINUTES HELPP
Answers
Answer:
In all of the ecoregions, wind and rain can weather and erode the landforms and soil. Rivers are also
powerful weathering and erosion agents. They can cut into rock and form canyons. When rivers deposit
eroded material, they change their shape or extend the shoreline. When waves along coastlines deposit
sediment, they extend beaches; when they wash sediment away, the waves erode beaches. Rivers, rock
formations, soils, topography, and precipitation in an ecoregion will determine how the processes of
weathering, erosion, and deposition work to reshape it.
Explanation:
how many molecules of H3PO4 are in 125 g of H3PO4
Answers
Answer:
2.11 * 10^3 molecules of phosphoric acid
Explanation:
how many moles of tin are there in 50 g of tin (II) oxide
Answers
Answer:
Moles of tin = 0.37 mol
Explanation:
Given data:
Mass of tin oxide = 50 g
Moles of tin = ?
Solution:
Number of moles of SnO:
Number of moles = mass/molar mass
Number of moles = 50 g/ 134.71 g/mol
Number of moles = 0.37 mol
We can see there is only one mole of Sn is present in one mole of tin oxide.
Thus in 0.37 mol of tin oxide,
0.37 mol × 1 = 0.37 mol of tin
(4.2 x 10^8) + (2.6 x 10^6) *
Answers
Answer:
4.226×10^8
Explanation:
4.2 ×10^8+2.6×10^6=422600000
or 4.226×10^8
An aerosol can contains 400.0 ml of compressed gas at 5.2 atm pressure. When the gas is sprayed into a large plastic bag, the bag inflates to a a volume of 2.14 L. What is the pressure of gas inside the plastic bag?
Answers
Answer:
0.97 atm ≅ 1 atm
Explanation:
The relation between the pressure (P) and volume (V) of a gas is given by the Boyle's law. According to this, the volume is inversely proportional to the pressure:
P₁ x V₁ = P₂ x V₂
From the problem, we have:
V₁= 400.0 mL x 1 L/1000 mL = 0.4 L
P₁= 5.2 atm
V₂= 2.14 L
We calculate the final pressure (P₂) from the equation, as follows:
P₂ = (P₁ x V₁)/V₂ = (5.2 atm x 0.40 L)/ 2.14 L = 0.97 atm ≅ 1 atm
Therefore, as the volume is increased (from 0.4 L to 2.14 L), the pressure in decreased (from 5.2 atm to 0.97 atm).
A 400.0-mL can contains compressed gas at 5.2 atm. The gas is sprayed into a bag, that inflates to 2.14 L at 0.97 atm.
What does Boyle's law state?Boyle's law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
A 400.0-mL can contains compressed gas at 5.2 atm. The gas is sprayed into a bag, that inflates to 2.14 L. We can calculate the final pressure of the gas using Boyle's law.
P₁ × V₁ = P₂ × V₂
P₂ = P₁ × V₁ / V₂
P₂ = 5.2 atm × 0.4000 L / 2.14 L = 0.97 atm
where,
P₁ and V₁ are the initial pressure and volume.P₂ and V₂ are the final pressure and volume.A 400.0-mL can contains compressed gas at 5.2 atm. The gas is sprayed into a bag, that inflates to 2.14 L at 0.97 atm.
Learn more about Boyle's law here: https://brainly.com/question/469270
help me pleaseeeee, if you do tyyy :)
Answers
How many grams are in 1.5moles of calcium
Answers
Answer: 60.117 g
Explanation: [tex]1.5 mol Ca = \frac{40.078g/mol}{1mol}[/tex] multiply and quit all "mol" and you get the grams
The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Which statement accurately describes a pattern in the size of atomic radii in the Periodic Table of the Elements?
*Atomic radii decrease from left to right across a period and decrease from top to
bottom in a group.
*Atomic radii increase from left to right across a period and increase from top to
bottom in a group.
*Atomic radii decrease from left to right across a period and increase from top to
bottom in a group.
*Atomic radii increase from left to right across a period and decrease from top to
Wottom in a group.
Answers
Answer:
The answer is: Atomic radii decreases from left to right across a period and increases from top to bottom in a group.
Explanation:
How much energy is required to melt 2 kg of copper? Use the table below and
this equation: Q = mLfusion:
Answers
414 kJ energy is required to melt 2 kg of copper. Hence, option B is correct.
What is energy?Energy is the capacity for doing work.
Given parameters:
Mass of copper = 2kg
Unknown:
Amount of energy required to melt the copper =?
Solution:
To solve this problem, we use:
Q = m L
m is the mass
L is the latent heat of fusion of copper = 207kJ/kg
So;
Q = 2 x 207 = 414kJ
Hence, option B is correct.
Learn more about energy here:
https://brainly.com/question/1932868
#SPJ2
Which of these is an example of a hypothesis?
Add different kinds of soil to three potted plants.
Observe fish swimming up a stream.
Temperature changes may be due to wind patterns.
Wonder why some layers of rocks contain fossils.
Answers
Explanation: because it “wonders”
The center atom of the CO 2 molecule corresponds to the notation
Answers
Answer:
simplest explanation regarding CO2 Molecular Geometry and hybridization. ... Carbon is the least electronegative that means it stays at the center
Explanation:
hope this helps
5.Summarize Which of the following is not an
advantage of using SI units?
A allows scientists to compare observations
and results
B can compare measurements made years
apart
C based on the number 5, which is easy to use
in calculations
D uses prefixes to express measurements that
are small or large
Answers
Answer:
C based on the number 5, which is easy to use in calculations
Explanation:
The SI unit system is not based on the number 5.
People with diabetes have to monitor and restrict their sugar intake. What volume of apple juice could
a diabetic person drink, if the person's sugar allowance for that beverage was 8.0 g? Assume that the
apple juice has a sugar concentration of 8.0 % w/v.
Answers
The volume of apple juice could a diabetic person drink : 100 ml
Further explanationGiven
sugar allowance = 8 g
sugar concentration of apple juice : 8.0% w/v
Required
Volume of apple juice
Solution
The concentration of a substance can be expressed in several quantities The concentration shows the amount of solute in a unit of the amount of solvent.
% w/v = mass of solute (weight) in g / 100 ml solution
So for 8% w/v = 8 g of sugar(solute) in 100 ml of apple juice
En un vaso se han puesto 250 g de alcohol junto con 2 g de yodo, que se disuelven completamente. Calcular la concentración de la disolución en % en masa.
Answers
The translation of the question is
250 g of alcohol have been put in a glass together with 2 g of iodine, which dissolve completely. Calculate the concentration of the solution in% by mass?
Answer:
The %mass by mass of the given solution is 0.794%
Explanation:
We are given
Mass of solute(Iodine) = 2g
Mass of the solvent (Alcohol) = 250g
Since the Iodine dissolves completely in Alcohol
Total mass of the solution = 250 + 2 = 252g
the formula of concentration of the solution in % by Mass is given by
%[tex]\frac{w}{W} = \frac{Mass of the solute*100}{Total mass of the solution}[/tex]
%w/W = [tex]\frac{2*100}{252}[/tex]
= 0.794%
Therefore the %mass by mass of the given solution is 0.794%
How does heat from the sun turn liquid water into gas called water vapor?
Answers
Answer:
Explanation:Once energy is added to water by heat from the sun, the bonds between the water molecules gain kinetic energy or energy in motion. Then they escape the surface of the liquid and become a gas (water vapor), which rises into atmosphere.
X-rays had a frequency of about 3x10^18 determine it's wavelength (in nm) and energy per photon
Answers
Answer:
λ = 0.1 nm
E = 2 × 10⁻¹⁵ J
Explanation:
Step 1: Given data
Frequency of X-rays (ν): 3 × 10¹⁸ s⁻¹
Step 2: Calculate the wavelength (λ) of a photon of X-rays
We will use the following expression.
c = λ × ν
where,
c: speed of light
λ = c / ν
λ = (3.00 × 10⁸ m/s) / 3 × 10¹⁸ s⁻¹
λ = 1 × 10⁻¹⁰ m × (10⁹ nm/1 m) = 0.1 nm
Step 3: Calculate the energy (E) of a photon of X-rays
We will use the following expression.
E = h × ν
where,
h: Planck's constant
E = 6.63 × 10⁻³⁴ J.s × 3 × 10¹⁸ s⁻¹
E = 2 × 10⁻¹⁵ J
On the graph, which dimension would be changed if the rate of reaction were to be altered by a catalyst?
Answers
Answer:
The alteration of the rate of the chemical reaction by the addition of a catalyst will change the dimension of L
Explanation:
The parts of the Energy profile of a reaction diagram the given letters represent are;
K = The energy possessed by the reactants
L = Activation energy required for the reaction to take place
M = Energy threshold required for the reaction to take place
N = The change in the enthalpy from the reactants to the products, which is also the energy released
O = The energy possessed by the products
The addition of a catalyst changes the route with which the reaction takes place such that the reaction rate is increased because the activation energy, L, is reduced.
Therefore, the dimension that will be changed if the rate of reaction were to be altered by a catalyst is L.
#1 - What is a pure substance? *
1 point
two or more atoms held together by chemical bonds
the attractive force that holds atoms together
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#2 - TRUE OF FALSE: Water is a pure substance.
1 point
True
False
#3 - What is an atom? *
1 point
the smallest unit of an element that keeps the properties of that element
the attractive force that holds atoms together
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#4 - What is a molecule? *
1 point
two or more atoms held together by chemical bonds
the attractive force that holds atoms together
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#5 - What is a chemical bond? *
1 point
two or more atoms held together by chemical bonds
the attractive force that holds atoms together
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#6 - TRUE OR FALSE: Molecules are all the same size. *
1 point
True
False
#7 - What is a compound? *
1 point
two or more atoms held together by chemical bonds
the attractive force that holds atoms together
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#8 - TRUE OR FALSE: Compounds never form in a fixed ratio. *
1 point
True
False
#9 - TRUE OR FALSE: The structure of matter at the atomic and molecular levels is too small to observe directly. *
1 point
True
False
#10 - TRUE OR FALSE: A complex molecule may contain a few atoms bonded together. A simple molecule may contain thousands of atoms. *
1 point
True
False
#11 - What is a crystal? *
1 point
two or more atoms held together by chemical bonds
a substance in which the particles are arranged in an orderly, geometric, and repeating pattern
a sample of matter that has specific chemical and physical properties
a pure substance made up of two or more different types of atoms joined by chemical bonds
#12 - TRUE OR FALSE: Diamond and graphite are both made of carbon atoms. *
1 point
True
False
Answers
Answer/Explanation:
#1 - A pure substance is either an element or a compound, making it different from a mixture. A sample of matter that has specific chemical and physical properties is the only one that fits here.
#2 - A pure substance is either an element or a compound, making it different from a mixture. Water is a compound, and thus this would be true.
#3 - An atom is the smallest unit of an element that keeps the properties of that element. Just refer to its definition.
#4 - A molecule is two or more atoms held together by chemical bonds . Again, just refer to its definition.
#5 - A chemical bond is the attractive force that holds atoms together . Jus refer to its definition.
#6 - Molecules are most definitely not all the same size, especially given the huge number of them. This would be false.
#7 - A compound is a pure substance made up of two or more different types of atoms joined by chemical bonds . Just refer to its definition.
#8 - Compounds always form in a fixed ratio, so this would be false.
#9 - If by directly, the problem means with the naked eye, this would be true.
#10 - False. It's reversed.
#11 - A crystal is a substance in which the particles are arranged in an orderly, geometric, and repeating pattern . Just refer to its definition.
#12 -True; to be more specific, they are covalent network solids.
what is a metorlogist
Answers
an expert in or student of meteorology; a weather forecaster.